Introduction
A 2012 article in The Lancet reported that four pain conditions ranked among the top 16 clinical afflictions affecting the world’s population [1]. Chronic pain has reached epidemic proportions in the United States, burdening the country, the people affected, and their families with medical costs, social isolation, and lost working hours [2]. With an aging population this burden will only get worse unless there are breakthroughs in medications and other treatments for chronic pain. At this time studies of effectiveness for chronic pain treatments suggest that 30 percent of patients have better results with some drugs than with placebos, which themselves tend to decrease pain by 20-30 percent. Currently, pharmacological pain treatment takes a trial-and-error approach; we treat on an ad hoc rotational system, starting with one drug and adding or replacing a drug on best-guess basis because we do not have specific diagnostic measures to pinpoint the cause of pain and few, if any, robust treatment approaches. We need a more objective, individualized process. So how can this change?
Why Is It So Hard to Objectify Pain?
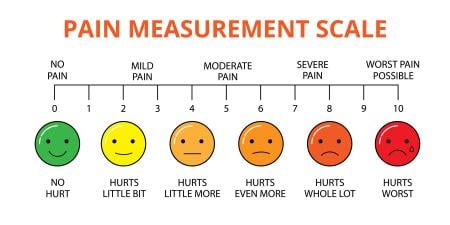
Nearly 100 million patients in the U.S. suffer from chronic pain. Chronic pain problems increase with age, and the U.S., as with other Western societies, is facing an increasingly large older population. All clinically active physicians will treat or interact with patients with chronic pain. Current approaches use a relatively simple metric—the 11-point pain scale (0 = no pain; 10 = maximal pain). But this does not capture the nature of chronic pain: a sensory and emotional response to an actual or perceived bodily threat that lasts for more than 3 months [3].
First, subjective responses, particularly for small differences, are notoriously difficult to evaluate, in part because pain means something different to each individual, in part because pain varies over time, and in part because pain is a complex experience that is also dependent on context, previous experience, social situation, psychological state, and so on. Second, the patient lives with chronic pain day in and day out, so capturing a chronic pain patient’s “state” as either a single measure or a measure over time is difficult. Third, no common diagnostic metric such as an EKG or blood tests for myocardial infarction exists.
Think about trying to describe a chronic pain process. The pain varies over time; pain is frequently comorbid with depression and anxiety (including posttraumatic stress disorder—PTSD); indeed it may induce these conditions in patients who were never depressed. Pain is a reward-deficit syndrome. Emotional, cognitive, and interoceptive (internal physiological status of self) components of pain are co-present. Even inattention and changes in perception of size are subtle but clear changes that are well described in chronic pain. Pain contributes to social isolation. Whatever its etiology, e.g., traumatic (e.g., postsurgical neuropathy), idiopathic (e.g., fibromyalgia), endocrine-related (e.g., diabetic neuropathy), due to an inborn error of metabolism (e.g., Fabry’s Disease), infection-related (e.g., post herpetic neuralgia), channelopathy-related (e.g., hemiplegic migraine, erythromelalgia), or other, chronic pain is a behavior and affects the brain and our response to the disease and its treatment.
Objective Metrics: From Genes to the Brain Systems
Technology is introducing several approaches to predicting the likelihood of chronic pain, measuring pain signals, and predicting response to analgesics. When patients are about to undergo surgery, diagnosis or prediction of the probability of chronic postsurgical neuropathic pain can be made using genetic [4], psychological [5], and imaging [6] measures.
Advances in nuclear magnetic resonance and functional activity measures allow brain pain signals to be visualized. Recent work has provided evidence that measures of brain structure and functional activity can define a pain state and predict disease chronification in conditions such as back pain [6] and perhaps migraine [7]. Some techniques have allowed for prediction of analgesic drug effects [8, 9].
These approaches are becoming more standardized, and routine use in clinical practice may not be far off. Specifically, the evolution of a brain biomarker for chronic pain (disease and analgesic effects) would seem to be well within grasp. If successful, these developments will contribute to transforming the poor state of chronic pain treatment. Brain imaging is one of many ongoing research areas that have radically changed our notion of chronic pain conditions. Other approaches to measures of brain systems in chronic pain include near-infrared spectroscopy (NIRS) and EEG methods that determine alterations in cortical changes. The latter have the benefit of being performed in the clinic and being cheaper.
Adapting to the Clinic
What would the characteristics of an objective measure or pain-metric be? A number of desired characteristics are obvious: (1) it should have high specificity and sensitivity; (2) it should be easy to implement—including testing procedures and evaluation processes (i.e., analysis, interpretation); and (3) the cost-benefit comparison should be clear. Simple approaches such as a questionnaire or a blood test are obviously preferable to complex methods like imaging. The more portable approaches noted above (NIRS, EEG) may be more cost-effective, but NMR imaging may acquire data faster and does not need application of specialized head caps for placement of electrodes or NIRS emitters and receivers.
Conclusions
We currently have no simple test of any kind that tells us whether someone has pain. Furthermore, we have few if any treatments that are highly effective in most patients with chronic pain [10]. Thus, the continued clinical challenge is to do the best we can for the individual patient using a therapeutic armamentarium that is by and large deficient or the efficacy of which is unclear.
If pain assessment based simply on the subjective rating worked well, we would probably have a more rational approach to treatment of our patients than the hit-and-miss approach that is generally practiced. Technology-based studies like those described above have not been replicated and validated sufficiently [11-14]. Most of them focused on cohorts of subjects and did not evaluate changes at the individual level. Nevertheless, there is reason to be optimistic that brain imaging can contribute to the overall evaluation of pain. The imaging field is in its infancy but the trend is towards metrics for individualized brain measures for pain and, for that matter, for other diseases that affect the brain, akin to the use of anatomical MRIs that is predicted to be part of routine clinical practice [13].
References
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9589):2163-2196.
-
Relieving Pain in America: A Blueprint for Transforming Preventing, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
- Costigan M, Belfer I, Griffin RS, et al. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133(9):2519-2527.
- Powell R, Johnston M, Smith WC, et al. Psychological risk factors for chronic post-surgical pain after inguinal hernia repair surgery: a prospective cohort study. Eur J Pain. 2012;16(4):600-610.
- Baliki MN, Petre B, Torbey S, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15(8):1117-1119.
-
Maleki N, Becerra L, Nutile L, et al. migraine attacks the basal ganglia. Mol Pain. 2011;7:71.
- Upadhyay J, Anderson J, Schwarz AJ, et al. Imaging drugs with and without clinical analgesic efficacy. Neuropsychopharmacology. 2011;36(13):2659-2673.
- Harris RE, Clauw DJ. Imaging central neurochemical alterations in chronic pain with proton magnetic resonance spectroscopy. Neurosci Lett. 2012;520(2):192-196.
-
Grosenick L, Klingenberg B, Katovich K, Knutson B, Taylor JE. Interpretable whole-brain prediction analysis with GraphNet. Neuroimage. 2013;72:304-321.
- Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia. Part 1: the need, reality, challenges, and solutions. Discov Med. 2011;11(58):197-207.
- Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia. Part 2: how, where, and what to look for using functional imaging. Discov Med. 2011;11(58):209-219.
-
Borsook D, Hargreaves R, Becerra L. Can functional magnetic resonance imaging improve success rates in CNS drug discovery? Expert Opin Drug Discov. 2011;6(6):597-617.
-
Tracey I. Can neuroimaging studies identify pain endophenotypes in humans? Nat Rev Neurol. 2011;7(3):173-181.
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. Engl J Med. 2013;368(15):1388-1397.