Diethylstilbestrol (DES) was first synthesized in 1938 and was the first orally active nonsteroidal estrogen that could be used for human therapy [1]. At that time, endocrinology was in its infancy and this discovery was a unique and great advance. Recurrent pregnancy loss was a serious medical problem then as it is now. It was believed the problems were due to a faulty hormonal environment of the fetal-placental unit, rather than primarily to genetic causes, as we have subsequently learned. There were studies at that time indicating that compromised pregnancies had a deficient output of the hormone progesterone, and further studies conducted in the late 1940s in Boston using very crude measuring techniques suggested that this deficiency could be corrected by administering DES to the mother, which would then lead to a healthy pregnancy. These studies led to the widespread usage of the drug to prevent pregnancy loss [2-4].
The initial examination of the newborns born to mothers treated with DES during pregnancy showed no abnormalities (C. Smith, personal communication). The treatment, however, was controversial. (A unique double-blind study conducted at the University of Chicago in the early 1950s failed to show any improved pregnancy outcome with DES therapy [5]. This study, while negative, was conducted on a healthy pregnant population, which was different than the Boston Study, which was conducted on a high-risk population with a history of bleeding in pregnancy or multiple pregnancy losses.) An additional problem is that the hormone assays in the Boston Study were later found to be faulty and not to actually measure progesterone (Louis L. Engel, PhD, personal communication). It is believed that DES continued to be heavily used in the 1960s, and it has been estimated that 2 to 4 million women in the United States took DES during pregnancy [6].
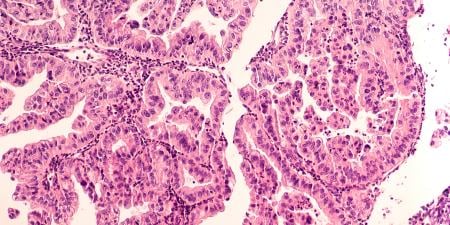
Then, in the late 1960s, eight extraordinarily rare cases of clear cell adenocarcinoma (CCA) of the vagina were diagnosed and treated in women in their teens and early 20s in the Boston area [7]. No such cluster of cases in young patients had ever been seen previously. CCA of the vagina was known to be a cancer that rarely occurred even in older women. In an effort to understand the cause of this cluster, a case-control study was conducted at the Massachusetts General Hospital in 1971 that linked the appearance of these cancers to the patients’ mothers having been treated with DES for pregnancy complications or having had a history of prior miscarriages [8]. This study resulted in the Food and Drug Administration’s (FDA) proscribing the use of DES for pregnancy support in 1971 [9]. To centralize data collection and to study the epidemiologic and clinical aspects of CCA of the vagina and cervix in DES-exposed young women, Dr. Robert E. Scully and I established the Registry for Research on Hormonal Transplacental Carcinogenesis in Boston in 1971 [10]. Further studies of patients with DES-associated CCA of the vagina and cervix showed that the cancers were rare among the DES-exposed, estimated to occur in about 1 per 1,000, with the average age of diagnosis being 19.0 years [11, 12].
Subsequently, DES use during pregnancy was associated with other adverse health effects in the exposed female offspring, including an increased frequency of anatomic problems in the female genital tract. These included cervicovaginal ridges, cervical hoods [13], and the underdevelopment of the cervix, all of which led to pregnancy complications including premature birth of offspring of the DES-exposed daughters [14]. Evaluations of DES-exposed daughters who did not have CCA showed a high prevalence of vaginal adenosis (benign glands in the vagina), and this finding was correlated with the time during pregnancy that the mother began DES treatment [15].Vaginal adenosis was also found to be associated with the development of vaginal CCA [16]. DES-exposed daughters were found to have abnormally shaped uteri, which led both to infertility problems and premature births of their offspring [17, 18]. Additional follow-up of DES-exposed mothers and daughters showed that each group appeared to have an increased risk of developing breast cancer [19-22].
Most recently, National Cancer Institute (NCI) collaborative studies showed that in-utero exposure of women to DES is associated with a high lifetime risk of a broad spectrum of adverse health outcomes, including an increased risk of breast cancer in daughters 40 years of age and older [22]. For DES-exposed sons, an increased risk of cancer has not been demonstrated [23] but they do have increased prevalence of maldescent of the testes, epididymal cysts, hypotrophic testes, and varicoceles [24-27]. This unique population continues to be studied long-term in a multi-institutional study by the National Cancer Institute, both to monitor the possible adverse health effects of DES in this population and to clarify which potential adverse effects are statistically significant and therefore of increased medical concern in this population. Some areas of concern include possible increased rates of cancers other than those cited previously, autoimmune diseases, and cardiac problems [28].
The DES granddaughters (third generation) are also being studied, and initial results showed that exposed granddaughters started their menstrual periods at a later age and were more likely to have irregular cycles than their unexposed peers, but they reported similar reproductive outcomes [29]. Thus far no other adverse health events have been demonstrated in this group. Regarding third generation DES-exposed sons, some studies have suggested an increased risk of hypospadias, a finding which has not been confirmed in subsequent studies [30, 31].
The CCA cases are currently being evaluated at the Registry for Research on Hormonal Transplacental Carcinogenesis. This registry, initially established in Boston [10], is now housed at the University of Chicago. Cases being accessioned are all cases of CCA of the vagina and cervix diagnosed in women born since 1948, whether or not there is a history of in-utero DES exposure. Thus far, more than 700 cases have been accessioned. Cases diagnosed at an early stage have been cured but cases diagnosed at a late stage have usually been fatal. About two-thirds of the cases have a history of exposure to DES [32] and most have developed in women under the age of 30 years, but the cancers are still occurring in older DES-exposed women, the oldest of whom was 62 years of age at the time of diagnosis. In addition, all cases of adenocarcinoma of the fallopian tube occurring in DES-exposed daughters are being accessioned.
This is a tragic example of a therapy that looked promising and was based on the best (but faulty) scientific evidence available at the time, which led to the widespread use of a treatment that the physician anticipated would help the patient have a successful pregnancy. However, due to the sensitivity of the developing fetus to an externally administered artificial hormone, unanticipated and severely adverse consequences developed. The physicians who prescribed DES were following what appeared to be accepted medical therapy that was based on published studies. Unfortunately, these studies were not based on the type of rigorous evaluation that is available today. There is still a risk of prescribing treatments that anecdotally appear promising but are not scientifically proven. This is a particular risk among infertility patients and obstetrical patients due to the “need” to have a positive outcome. The DES saga, in the opinion of the authors, provides a strong reason to support the use of carefully constructed scientific trials to evaluate new therapies, and this admonition is particularly applicable to the pregnant patient, since the fetus is often more sensitive than the living offspring to outside influences.
References
-
Dodds EC, Goldberg L, Larson W, Robinson R. Oestrogenic activity of certain synthetic compounds. Nature. 1938;141:247-248.
- Smith OW. Diethylstilbestrol in the prevention and treatment of complications of pregnancy. Am J Obstet Gynecol. 1948;56(5):821-834.
-
Smith OW, Smith GV, Hurwitz D. Increased excretion of pregnanediol in pregnancy from diethylstilbestrol with special reference to the prevention of late pregnancy accidents. Am J Obstet Gynecol. 1946;51:411-415.
- Smith OW, Smith GV. The influence of diethylstilbestrol on the progress and outcome of pregnancy as based on a comparison of treated with untreated primigravidas. Am J Obstet Gynecol. 1949;58(5):994-1009.
-
Dieckmann WJ, Davis ME, Rynkiewicz LM, Pottinger RE. Does the administration of diethylstilbestrol during pregnancy have therapeutic value? Am J Obstet Gynecol. 1953;66(5):1062-1081.
-
Fink DJ. DES Task Force summary report. Bethesda, MD: National Institutes of Health; 1978.
- Herbst AL, Scully RE. Adenocarcinoma of the vagina in adolescence. A report of 7 cases including 6 clear cell carcinomas (so-called mesonephromas). Cancer. 1970;25(4):745-757.
- Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284(15):878-881.
-
Selected item from the FDA Drug Bulletin—November 1971: diethylstilbestrol contraindicated in pregnancy: drug’s use linked to adenocarcinoma in the offspring. Calif Med. 1972;116(2):85-86.
- Herbst AL, Kurman RJ, Scully RE, Poskanzer DC. Clear-cell adenocarcinoma of the genital tract in young females. Registry Report. N Engl J Med. 1972;287(25):1259-1264.
- Herbst AL, Cole P, Colton T, Robboy SJ, Scully RE. Age-incidence and risk of diethylstilbestrol-related clear cell adenocarcinoma of the vagina and cervix. Am J Obstet Gynecol. 1977;128(1):43-50.
- Melnick S, Cole P, Anderson D, Herbst A. Rates and risks of diethylstilbestrol-related clear-cell adenocarcinoma of the vagina and cervix. An update. N Engl J Med. 1987;316(9):514-516.
- Herbst AL, Kurman RJ, Scully RE. Vaginal and cervical abnormalities after exposure to stilbestrol in utero. Obstet Gynecol. 1972;40(3):287-298.
- Kaufman RH, Noller K, Adam E. Upper genital tract abnormalities and pregnancy outcome in diethylstilbestrol-exposed progeny. Am J Obstet Gynecol. 1984;148(7):973-984.
- Herbst AL, Poskanzer DC, Robboy SJ, Friedlander L, Scully RE. Prenatal exposure to stilbestrol. A prospective comparison of exposed female offspring with unexposed controls. N Engl J Med. 1975;292(7):334-339.
- Robboy SJ, Welch WR, Young RH, Truslow GY, Herbst AL, Scully RE. Topographic relation of cervical ectropion and vaginal adenosis to clear cell adenocarcinoma. Obstet Gynecol. 1982;60(5):546-551.
- Kaufman RH, Adam E, Noller K, Irwin JF, Gray M. Upper genital tract changes and infertility in diethylstilbestrol-exposed women. Am J Obstet Gynecol. 1986;154(6):1312-1318.
- Herbst AL. Diethylstilbestrol and other sex hormones during pregnancy. Obstet Gynecol. 1981;58(5)(suppl):35S-40S.
- Titus-Ernstoff L, Hatch EE, Hoover RN, et al. Long-term cancer risk in women given diethylstilbestrol (DES) during pregnancy. Brit J Cancer. 2001;84(1):126-133.
- Palmer JR, Hatch EE, Rosenberg CL, et al. Risk of breast cancer in women exposed to diethylstilbestrol in utero: preliminary results (United States). Cancer Causes Control. 2002;13(8):753-758.
- Palmer JR, Wise LA, Hatch EE, et al. Prenatal diethylstilbestrol exposure and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1509-1514.
- Hoover RN, Hyer M, Pfeiffer RM, et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med. 2011;365(14):1304-1314.
- Strohsnitter WC, Noller KL, Hoover RN, et al. Cancer risk in men exposed in utero to diethylstilbestrol. J Natl Cancer Inst. 2001;93(7):545-551.
- Gill WB, Schumacher GF, Bibbo M, Straus II FH, Schoenberg HW. Association of diethylstilbestrol exposure in utero with cryptorchidism, testicular hypoplasia and semen abnormalities. J Urol. 1979;122(1):36-39.
- Leary FJ, Resseguie LJ, Kurland LT, O’Brien PC, Emslander RF, Noller KL. Males exposed in utero to diethylstilbestrol. JAMA. 1984;252(21):2984-2989.
- Wilcox AJ, Baird DD, Weinberg CR, Hornsby PP, Herbst AL. Fertility in men exposed prenatally to diethylstilbestrol. N Engl J Med. 1995;332(21):1411-1416.
-
Palmer JR, Herbst AL, Noller KL, et al. Urogenital abnormalities in men exposed to diethylstilbestrol in utero: a cohort study. Environ Health. 2009;8:37.
- Troisi R, Hyer M, Hatch EE. Medical conditions among adult offspring prenatally exposed to diethylstilbestrol. Epidemiol. 2013;24(3):430-438.
- Titus-Ernstoff L, Troisi R, Hatch EE, et al. Menstrual and reproductive characteristics of women whose mothers were exposed in utero to diethylstilbestrol (DES). Int J Epidemiol. 2006;35(4):862-868.
-
Klip H, Verloop J, van Gool JD, et al; OMEGA Project Group. Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study. Lancet. 2002;359(9312):1102-1107.
- Palmer JR, Wise LA, Robboy SJ, et al. Hypospadias in sons of women exposed to diethylstilbestrol in utero. Epidemiology. 2005;16(4):583-586.
- Palmer JR, Anderson D, Helmrich SP, Herbst AL. Risk factors for diethylstilbestrol-associated clear cell adenocarcinoma. Obstet Gynecol. 2000;95(6, pt 1):814-820.