Abstract
Robotic-assisted techniques in surgery require complex equipment setup and create surgeon isolation through loss of normal operative visual and auditory exchanges. These changes demand enhanced team communication and feedback in operating room settings so that robotic-assisted surgery does not compromise safe, efficient surgical care. Diverse simulation and training methods should be incorporated and studied based on local needs to determine how these techniques influence operating room communication, workflow, and safety culture.
Curricular Evolution
Although robots were used in the mid-1980s to assist in computed tomography-guided brain biopsy,1 robotic-assisted technology rapidly expanded within urologic and gynecologic surgery in the early 2000s and was used with increasing frequency in general surgery in the 2010s.2,3 In response to this rapid growth, many robotic training programs and curricula have been developed to expose surgeons, trainees, and operating room team members to the unique features of robotic-assisted surgery. Online learning modules to introduce robotic surgical systems, for example, focus on providing general knowledge, such as device function, instrumentation, and troubleshooting. These programs and curricula have been coupled with bedside-training opportunities and task practice on robotic surgery simulators for psychomotor skill development.4,5
Despite these initial focused efforts, surgeons and surgical teams still face both technical and nontechnical challenges in the live operating room setting that pose barriers to performance of safe and efficient robotic-assisted procedures. More complex robotic preparation and equipment, for example, increase the opportunity for surgical flow disruptions to occur in the operating room. Physical disruptions, such as incorrect placement of carts and consoles, equipment collisions, and other issues, are common and occur more frequently in robotic-assisted approaches than when using other techniques.6,7 Furthermore, communication disruptions have been associated with worse surgical outcome metrics for patients, such as longer operative times and higher estimated blood losses.8 Poor nontechnical skills in robotic surgery teams, such as ineffective verbal communication, limited situational awareness of the patient’s condition, and poor delegation and coordination of tasks have also been linked with increased near-miss events during operative cases.9
Given these new concerns, the surgical community must determine how best to train for and use robotic-assisted surgery in ways that maintain patient safety and efficacy. Herein we describe the unique physical and communication features of robotic-assisted surgery that have impacted operating room culture and discuss potential strategies for optimizing these features in the future to best promote a culture of safety.
Unique Challenges
To initiate the active operative component of a robotic-assisted surgical case, several new steps are necessary that require the entire operating room team to participate in the setup, preparation, and docking of the robotic system. First, the system must be sterilely draped and positioned appropriately with respect to the patient and operating table. Next, robotic arms are positioned in a procedure-specific orientation, and the circulating team carefully moves the patient cart to the correct location over the patient. Some teams then use the target function of the robotic platform to confirm and adjust this positioning. Finally, robotic arms are docked to respective surgical trocars so that appropriate surgical instruments can be inserted and ready for use. Lack of communication or familiarity with the steps of this process can add significant time to the setup phase of the procedure. However, with proper team training and experience over time, the setup can be significantly streamlined with minimal additional risk posed to the patient. Several studies have now shown acceptable robotic preparation and docking times, which improve as team members gain experience.10,11
Following the initial setup, the surgeon and surgical trainees physically separate from the sterile operative field by stepping away from the patient’s bedside to begin operating at the robotic console. This separation is required for functioning of the robotic-assisted system and is an act that distinguishes robotic-assisted surgery from other modalities. The robotic console is typically positioned several feet away from the bedside assistant as well as the scrub and circulating members of the team. When surgeons place their heads in the console, they gain an immersive, high-resolution, 3D view of the operative field as well as other advantages, such as improved ergonomics and dexterity. More recently, many academic health centers have also acquired the dual-console robotic system, which allows the proctoring or teaching faculty surgeon to experience these benefits while participating in parallel with the training surgeon away from the patient’s bedside in the operating room. The dual setup has been instrumental in allowing safe, graded involvement of the training surgeon, as well as easier instrument handoffs and use of features such as the virtual pointer tool to help facilitate intraoperative teaching.12,13
As surgeons and trainees immerse themselves in the robotic console, however, the physical separation or gap between surgeons and team members can lead to surgeon isolation and loss of situational awareness, defined as an accurate understanding of all patient and environmental factors throughout a procedure in the operating room.14 Physical positioning of the console away from the patient prevents the surgeon from being able to globally observe what is happening in the operating room and to make visual contact with bedside, nursing, and anesthesia teams.15 Common intraoperative occurrences, such as team members stepping out of the operating room to retrieve equipment or during a shift change, may go unnoticed if not verbally announced. Console immersion or tunnel vision may also prevent a potential patient safety concern from being properly identified.16 For example, surgeons with their head positioned in the console see a visual feed from the camera and robotic instruments but cannot immediately visualize equipment collisions and malfunctions or identify changes in patients’ hemodynamic monitoring without removing themselves from this console “head-in” position. With the surgeon physically separated from the rest of the team, it is also more difficult for the surgeon to coordinate team actions that may be needed to address these issues and move the case forward safely.17 In addition, team members who are assisting at the bedside for prolonged cases often become disengaged due to this separation, again decreasing situational awareness.16
Console immersion or tunnel vision may prevent a potential patient safety concern from being properly identified.
Beyond the physical challenges, communication using the robotic system also presents unique difficulties for operating room team members. First, though dual console operators communicate with each other throughout the case using a built-in microphone system, their commentary and requests are often difficult for the rest of the room to hear, given variable sound transmission and muffled speakers.16,18 This microphone system would benefit from a technical redesign that allows better amplification of bidirectional communications between surgeons and their teams. The team’s inability to hear surgeons’ instructions and vice versa can result in disorganized assistance being provided at the patient’s bedside, including exchange of incorrect robotic instruments at relevant points in the case. Instances of poor communication and subsequent surgical flow disruptions contribute to delays in forward case progression for patients. Repeated or unacknowledged requests can also lead to surgeon frustration and subsequent team conflicts.19 Given the additional loss of nonverbal cues and exchanges between team members secondary to the robotic spatial configuration, a focus on improving the verbal communication component of these cases is of utmost importance to promote increased safety in robotic operating rooms.20
Potential Solutions
To improve successful integration of robotic technology, decrease potential harmful robotic workflow disruptions, and ideally overcome the challenges discussed, several interventions have been proposed and studied.
Interprofessional team training. Interprofessional team training and simulation are promising methods for increasing situational awareness. Heightened situational awareness is particularly critical in robotics, given the tunnel vision effect of the console. Interprofessional simulation training involving surgeons and nurses has previously been shown to decrease robotic system docking times, mean operative times, and mean costs per case.21 Participation in game-based educational competitions has also been shown to help robotic team members cultivate an understanding and a culture of teamwork through use of interactive learning environments.22
Interdisciplinary training has the potential to help team members practice their individual roles while the team reviews its functioning as a collective unit. In interdisciplinary training, surgeons, trainees, anesthesiologists, as well as scrub and circulating nurses, jointly participate in hands-on workshops and simulations to practice operating room and robotic system setup, emergency scenarios, and communication strategies, such as time-outs and debriefings.21 Such trainings can enhance team performance and streamline workflow to minimize physical and communication disruptions that are unique to robotic surgery. New assessment tools, such as the Robotic-Assisted Surgery Oxford Non-Technical Skills observational tool, have also been developed for assessment of teamwork behaviors in robotic surgery.23 This tool can now be used before and after team training events to determine which interventions are most impactful. It can also be applied in live operative procedures to better characterize strengths and weaknesses of the operative team overall for future, focused improvement efforts.
Patient emergency training. Given robotic surgery’s potential risks to patient safety, other approaches have focused specifically on team functioning in the event of patient emergency. Curricula to train emergency robotic undocking procedures have been shown to improve undocking times and performance of critical actions for operative teams.24,25 Emergency simulations have additionally promoted improvement of robotic team members’ nontechnical skills, such as situational awareness, decision making, leadership, communication, and teamwork.26 It is critical that hospitals with robotic capabilities develop emergency safety protocols and provide relevant training and simulation opportunities to support cohesive responses in life-threatening patient events. Emergency safety protocols can reduce both errors and process disruptions in such high-pressure scenarios.27
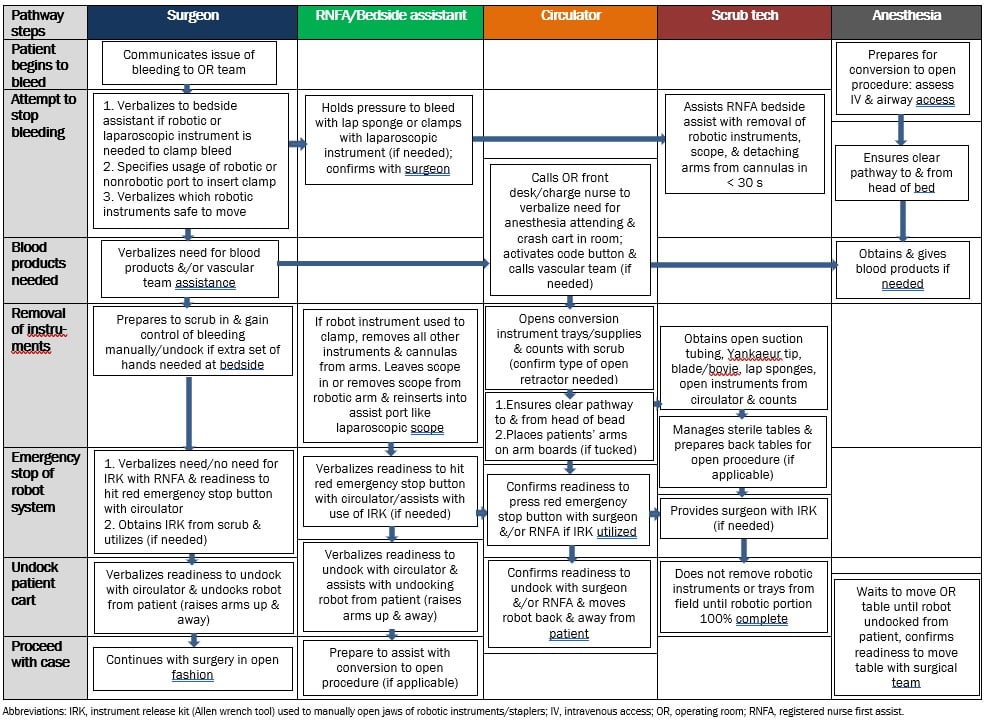
At our own institution, we have developed emergency undocking protocols for specific occurrences, such as life-threatening intraoperative bleeding and patient code events (see Figure). These protocols are organized by “swim lanes” to delineate team roles and duties to be performed in parallel during the emergency event. For example, as the surgeon and bedside assistant attempt to control bleeding with robotic instruments, the circulator alerts the operating room front desk and charge nursing team of the situation, and the anesthesia team assesses the patient for appropriate intravenous access should blood products be required. These protocols have been tested with robotic teams in our simulation center and refined for larger-scale group training and assessment. Although our focus has been on intraoperative bleeding and code events, the algorithmic approach could be applied to other scenarios, such as intraoperative anaphylaxis or cardiac events, and even to technical system malfunctions.
Figure. Barnes-Jewish Hospital/Washington University Emergency Undocking Bleeding Protocol
Although many efforts have focused on improving robotic operative culture and teamwork, there is still a need for routine, robotic-specific communication practices. Training in closed-looped communication may help to promote increased patient safety. This strategy focuses on simple and standardized procedures for message transmission, reception, and verification between team members.28 Read-back techniques in closed loop communication, wherein the team member receiving information verbally repeats it back to the sender, have been shown to increase information transfer among members of health care teams in other settings29 but have not been explicitly studied in robotic surgery. Preparing each robotic case with a briefing, a reminder of docking requirements, delineation of specific roles, and read-backs after completion of key tasks could increase team efficiency and coordination.5 This preparation may also help to overcome visual and auditory challenges that are unique to robotic surgery. Although several checklists have been developed to guide this process in robotic simulated and live operative environments,30,31 these checklists require further study to determine their effectiveness when implemented in routine robotic cases.
Conclusion
The introduction of robotic technology in surgery has allowed for innovation while creating several new challenges that teams must overcome to provide safe and efficient surgical care. Diverse simulation and training methods have been studied and should be incorporated within institutions based on local needs to minimize workflow disruptions and potential patient safety events. Highly motivated operative teams that encourage a culture of teamwork will be most successful in overcoming these challenges and closing the physical and communication gaps attendant on robotic-assisted surgery in the future.
References
- Siepel FJ, Maris B, Welleweerd MK, Groenhuis V, Fiorini P, Stramigioli S. Needle and biopsy robots: a review. Curr Robot Rep. 2021;2(1):73-84.
- Juo YY, Mantha A, Abiri A, Lin A, Dutson E. Diffusion of robotic-assisted laparoscopic technology across specialties: a national study from 2008 to 2013. Surg Endosc. 2018;32(3):1405-1413.
-
Sheetz KH, Claflin J, Dimick JB. Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw Open. 2020;3(1):e1918911.
-
Chen R, Rodrigues Armijo P, Krause C, Siu KC, Oleynikov D; SAGES Robotic Task Force. A comprehensive review of robotic surgery curriculum and training for residents, fellows, and postgraduate surgical education. Surg Endosc. 2020;34(1):361-367.
- Wang RS, Ambani SN. Robotic surgery training: current trends and future directions. Urol Clin North Am. 2021;48(1):137-146.
- Catchpole K, Perkins C, Bresee C, et al. Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc. 2016;30(9):3749-3761.
- Cofran L, Cohen T, Alfred M, et al. Barriers to safety and efficiency in robotic surgery docking. Surg Endosc. 2022;36(1):206-215.
-
Schiff L, Tsafrir Z, Aoun J, Taylor A, Theoharis E, Eisenstein D. Quality of communication in robotic surgery and surgical outcomes. JSLS. 2016;20(3):e2016.00026.
- Manuguerra A, Mazeaud C, Hubert N, et al. Non-technical skills in robotic surgery and impact on near-miss events: a multi-center study. Surg Endosc. 2021;35(9):5062-5071.
- Iranmanesh P, Morel P, Wagner OJ, Inan I, Pugin F, Hagen ME. Set-up and docking of the da Vinci surgical system: prospective analysis of initial experience. Int J Med Robot. 2010;6(1):57-60.
- van der Schans EM, Hiep MAJ, Consten ECJ, Broeders IAMJ. From da Vinci Si to da Vinci Xi: realistic times in draping and docking the robot. J Robot Surg. 2020;14(6):835-839.
- Fernandes E, Elli E, Giulianotti P. The role of the dual console in robotic surgical training. Surgery. 2014;155(1):1-4.
- Zhao B, Lam J, Hollandsworth HM, et al. General surgery training in the era of robotic surgery: a qualitative analysis of perceptions from resident and attending surgeons. Surg Endosc. 2020;34(4):1712-1721.
- O’Dea A, Morris M, O’Keeffe D. Experiential training for situation awareness in the operating room. JAMA Surg. 2022;157(1):66-67.
-
Randell R, Alvarado N, Honey S, et al. Impact of robotic surgery on decision making: perspectives of surgical teams. AMIA Annu Symp Proc. 2015;2015:1057-1066.
- El-Hamamsy D, Walton TJ, Griffiths TRL, Anderson ES, Tincello DG. Surgeon-team separation in robotic theaters: a qualitative observational and interview study. Female Pelvic Med Reconstr Surg. 2020;26(2):86-91.
-
Randell R, Honey S, Hindmarsh J, et al. A Realist Process Evaluation of Robot-Assisted Surgery: Integration Into Routine Practice and Impacts on Communication, Collaboration and Decision-Making. NIHR Journals Library; 2017.
- Cristofari H, Jung MK, Niclauss N, Toso C, Kloetzer L. Teaching and learning robotic surgery at the dual console: a video-based qualitative analysis. J Robot Surg. 2022;16(1):169-178.
- Catchpole KR, Hallett E, Curtis S, Mirchi T, Souders CP, Anger JT. Diagnosing barriers to safety and efficiency in robotic surgery. Ergonomics. 2018;61(1):26-39.
- Almeras C, Almeras C. Operating room communication in robotic surgery: place, modalities and evolution of a safe system of interaction. J Visc Surg. 2019;156(5):397-403.
- Vigo F, Egg R, Schoetzau A, et al. An interdisciplinary team-training protocol for robotic gynecologic surgery improves operating time and costs: analysis of a 4-year experience in a university hospital setting. J Robot Surg. 2022;16(1):89-96.
- Cohen TN, Anger JT, Kanji FF, et al. A novel approach for engagement in team training in high-technology surgery: the robotic-assisted surgery Olympics. J Patient Saf. 2022;18(6):570-577.
- Schreyer J, Koch A, Herlemann A, et al. RAS-NOTECHS: validity and reliability of a tool for measuring non-technical skills in robotic-assisted surgery settings. Surg Endosc. 2022;36(3):1916-1926.
- Melnyk R, Saba P, Holler T, et al. Design and implementation of an emergency undocking curriculum for robotic surgery. Simul Healthc. 2022;17(2):78-87.
-
Ballas DA, Cesta M, Gothard D, Ahmed R. Emergency undocking curriculum in robotic surgery. Cureus. 2019;11(3):e4321.
-
Fabrício Pereira de Almeida T, Esteves Chaves Campos M, Romanelli de Castro P, et al. Training in the protocol for robotic undocking for life emergency support (RULES) improves team communication, coordination and reduces the time required to decouple the robotic system from the patient. Int J Med Robot. 2022;18(6):e2454.
- Kalipershad SNR, Peristerakis I. The introduction of an emergency safety protocol coupled with simulation training in robotic surgery, has enabled a more cohesive and efficient response to emergencies. Surgeon. 2022;20(3):151-156.
-
Härgestam M, Lindkvist M, Brulin C, Jacobsson M, Hultin M. Communication in interdisciplinary teams: exploring closed-loop communication during in situ trauma team training. BMJ Open. 2013;3(10):e003525.
- Boyd M, Cumin D, Lombard B, Torrie J, Civil N, Weller J. Read-back improves information transfer in simulated clinical crises. BMJ Qual Saf. 2014;23(12):989-993.
- Garg T, Bazzi WM, Silberstein JL, Abu-Rustum N, Leitao MM Jr, Laudone VP. Improving safety in robotic surgery: intraoperative crisis checklist. J Surg Oncol. 2013;108(3):139-140.
- Ahmed K, Khan N, Khan MS, Dasgupta P. Development and content validation of a surgical safety checklist for operating theatres that use robotic technology. BJU Int. 2013;111(7):1161-1174.