Abstract
Bats are diverse mammals that are globally distributed and ecologically critical, yet some bat species are associated with disease agents that have severe consequences for human health. Disease outbreak responses require interdisciplinary knowledge of bat-associated pathogens and microbial transmission patterns. Health promotion requires close, collaborative attention to the needs, vulnerabilities, and interests of diverse stakeholders, including the public and professionals in public health, conservation, ecology, social science, communication, and policy. This article describes a successful One Health engagement among such stakeholders and partners looking to motivate both bat-human ecology preservation and viral disease management in Uganda.
Convergence and Disease
Zoonotic infectious disease emergence is a complex process that necessitates understanding the vertebrate reservoir ecology; circulation, shedding, and maintenance of the infectious agent in its natural reservoir host; reservoir-human or environmental contacts that may lead to human exposure; and intrahost immunological barriers that may protect against infection.1 This convergence of human, animal, and environmental health in the context of infectious disease dynamics underpins One Health, which the Centers for Disease Control and Prevention defines as “a collaborative, multisectoral, and transdisciplinary approach—working at the local, regional, national, and global levels—with the goal of achieving optimal health outcomes [by] recognizing the interconnection between people, animals, plants, and their shared environment.”2 The One Health framework has become a cornerstone of global infectious disease investigations,3,4,5,6 building on a growing appreciation for how changing landscapes, human encroachment onto pristine habitats, and interconnected global travel facilitate the spread of infectious agents.
One Health provides an interdisciplinary framework for understanding the nature of initial spillover events and mitigating subsequent pathogen transmission. Significant contributions from animal health, infectious disease, environmental health, human health, and governmental sectors, as well as interdisciplinary engagement among these groups, are essential to a comprehensive mission with One Health at the center (see Figure). From the perspective of studying the viral ecology of bats in Uganda, we posit that interdisciplinary approaches within this One Health framework are essential to the successful investigation and response to zoonotic infectious disease events, and we present key opportunities for effective cross-disciplinary engagement in this context.
Figure. Conceptual Interdisciplinary Framework for One Health Investigations on Zoonotic Emerging Infectious Diseases
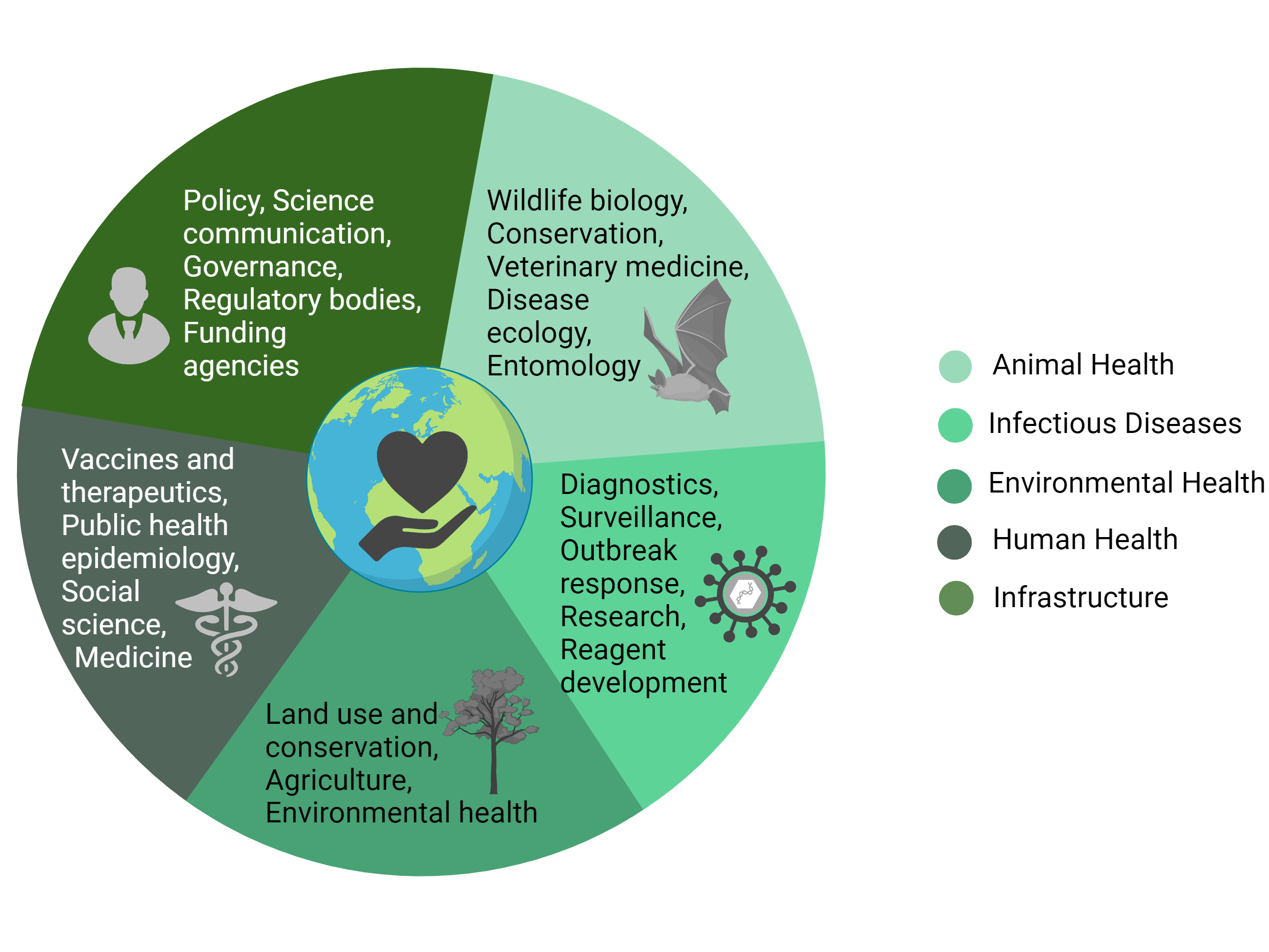
Created with BioRender.com.
Animal Health
By occupying a diverse range of ecological niches, bats provide numerous and critical ecological services, such as pest insect consumption, seed dispersal, and pollination.7 Yet some bat species are associated with highly consequential human pathogenic viruses, including lyssaviruses,8,9 coronaviruses,10,11 paramyxoviruses,12,13 and filoviruses.14 The fear of bats and the negative association of emerging diseases with bats threaten the wildlife conservation mission and raise issues about animal welfare, despite wildlife conservation being integral to One Health.15 In Uganda, for example, miners’ fear of Marburg virus triggered a mass culling of Egyptian rousette bats in a mine in 2008.16 However, by 2012, bats had recolonized the mine, and Marburg infection rates were higher than during the 2007 to 2008 outbreak.16 Similarly, the government of Mauritius ordered multiple mass cullings of the endangered Mauritius fruit bat (Pteropus niger), reportedly due to fruit crop damage.17
While this discordance between conservation goals and infectious disease control has created tension within the bat research community,18 a One Health perspective recognizes that the protection of bats and their habitats from anthropogenic disturbance and the protection of humans from zoonotic disease function together as one interconnected system.15 For example, understanding how viruses circulate in bat populations provides insight into both drivers of pathogen shedding as it relates to human health risk and potential risks to bat health from human encroachment and bats’ interactions with domestic animals. Thus, wildlife professionals’ insights on bat ecology are essential to protecting bats and mitigating risks to people and livestock. In particular, wildlife veterinarians applying a One Health framework are in a unique position to act as liaisons between human medical professionals and wildlife biologists, whose interests have rarely overlapped previously.19
In the ecological system the authors study in Uganda, peri-domestic animals can have contact with cave-dwelling bats and with bat waste, including feces, urine, and partially eaten fruits. Cattle, goats, dogs, and various wildlife species enter caves for salt or other micronutrients, for protection, and possibly to hunt or scavenge bats. These species may then have contact with other domestic animals or people, directly or indirectly, creating a potential chain of contact by which infectious agents could be transmitted from one species to another. Moreover, that bat roost sites often have no deliberate protection and increasingly are encroached upon by human settlements raises concerns about increased human-bat contact.20 The senior author (R.C.K.) and a colleague have argued that understanding and mitigating potentially complex spillover events from bats can be facilitated through a One Health emphasis on open communication and interdisciplinary collaboration among bat ecologists and infectious disease researchers.18 Namely, identifying how existing research programs can be leveraged to further common agendas, building new research domains that address questions fundamental to both disciplines (eg, bat health), and strengthening relationships to facilitate knowledge transfer can all break down barriers to interdisciplinary collaboration.18 International organizations such as the Global Union of Bat Diversity Networks (GBatNet)21 and the International Union for Conservation of Nature Bat Specialist Group22 have already made great strides in facilitating interdisciplinary, action-oriented engagements between bat researchers focused on conservation and on infectious diseases. For example, GBatNet has assembled a number of interdisciplinary working groups to tackle cross-cutting One Health issues, including bat health and stress, science communication, and the impact of climate change.21
Human Health
Medical professionals are expert at early recognition of atypical disease presentations as well as characteristic signs and symptoms, case definition development, treating patients, evaluating vaccines and therapeutics, and communicating with public health agencies and regulatory stakeholder groups, but they are only one part of a One Health approach to infectious disease control. Before One Health, One Medicine recognized commonalities between human and veterinary medicine’s shared epidemiological approaches to combatting human and veterinary diseases and called for collaboration to address zoonotic disease.23 The need for a collaborative approach to achieve this goal is apparent in Uganda, where human-bat-cave interactions have resulted in disease outbreaks,13,14,16 but rural disease reporting capabilities are limited. Despite having a village health team (VHT) network for community disease surveillance, volunteers are challenged by inadequate access to transportation for surveillance data collection; lack of data analysis and storage capacity; no coordination among environmental, wildlife, or livestock surveillance; and inability to use mobile phones for data reporting.24 Experts’ engagement with communities is essential for early recognition and response to emerging infectious disease events. While Uganda has formalized a National One Health Platform for advising government agencies and coordinating their responses to zoonotic diseases, this endeavor lacks financial backing and actual intersectoral coordination.25 Such cross-agency coordination of One Health surveillance activities that include VHTs would be game changing for the transmittal of disease information from communities to district and national task forces responsible for rapid epidemic and emergency response.24,25 Moreover, interdisciplinary collaboration is key to understanding and mitigating spillover events, as collaboration among physicians, veterinarians, and wildlife biologists enables clinical disease symptoms to be traced to unusual bat exposure events.13 Similarly, social scientists and anthropologists can work with disease ecologists and wildlife researchers to determine human behaviors and interactions with reservoir hosts or vectors that predispose people to zoonoses and may facilitate the emergence of zoonotic spillover events,26 thereby enabling the development of targeted intervention strategies (eg, educational campaigns in schools and training workshops for district officials).
Environmental Health
With less than 3% of the earth’s terrestrial surface qualifying as “intact,”27 there is increasing need for cross-disciplinary collaborations to ensure healthy environments for all species. Environmental changes associated with urbanization, human population growth, increasing demand for animal protein, intensive farming systems, unsustainable natural resource consumption, biodiversity loss, and habitat fragmentation all contribute to the emergence and spillover of infectious diseases primarily through enhanced human-wildlife interactions.28 How can we ensure that vulnerable communities experience food and financial security without destabilizing habitats in a way that creates new risk for zoonotic disease emergence? This intersection of social and environmental sciences raises questions about how best to support the health and prosperity of rural impoverished communities living in or adjacent to fragile, protected ecosystems.
Bats in protected and unprotected areas face increased anthropogenic disturbance, which threatens the natural buffers of our ecosystems, causing a disruption in the flow of ecosystem services.
In Uganda as elsewhere, the proportion of land area under some form of protection is small for the size of the country and is shrinking due to human activity,29 which has consequences for the environment and for human and animal health. Natural land cover is important for maintaining healthy communities and populations of bats; such habitats preserve ecosystem function and limit human-bat interaction. Bats in protected and unprotected areas, however, face increased anthropogenic disturbance, which threatens the natural buffers of our ecosystems, causing a disruption in the flow of ecosystem services. Moreover, the encroachment of human settlements into areas where bats may be foraging for fruits or insects increases the potential for human-bat contact and raises concerns about spillover risk.20 The increased environmental stress can also impair wildlife immunity, causing increased shedding of pathogens to the environment, where humans and animals may be exposed.30 Thus, habitat conservation should be the aim of multidisciplinary efforts to protect bat populations and to limit bat interactions with people and thereby minimize spillover risk of bat-borne pathogens. As provided for under Section 35(1)(f) of the Uganda Wildlife Act of 2019,31 local communities living along the Mt Elgon National Park border are permitted to enter the park weekly to sustainably harvest resources such as medicinal plants, mushrooms, grass for thatching houses, fish, and fuel wood as one of the solutions for promoting environmental and public health protection.
Infrastructure and Infectious Diseases
It is imperative to have highly trained infectious disease scientists and a laboratory infrastructure to support safe zoonotic disease investigations, biosecurity during research, and emergency response efforts. In Uganda, the Uganda Virus Research Institute (UVRI), founded in 1936—one of the premiere infectious disease institutions in Africa—subserves these functions. At UVRI, more than 124 virus strains of 14 new viruses have been isolated, including West Nile virus and bat-associated viruses.32 The UVRI conducts research, surveillance, and diagnostic activities and serves as a World Health Organization reference and testing laboratory.33
Recently, an international collaboration led to the development of the Laboratory Response Checklist for Infectious Disease Outbreaks.34 This checklist specifies that outbreak investigative teams—comprising epidemiologists, laboratory diagnosticians, wildlife professionals, risk analysts, and infectious disease specialists—should provide extensive interagency and institutional coordination among stakeholder groups to implement disease surveillance and effective response measures. Such coordination ensures that viable samples are safely collected from humans, animals, and the environment to monitor causative pathogens. The outbreak data collected should be analyzed to discover behavioral dynamics, clustering patterns, and disease hotspots, and molecular and phylogenetic studies should be carried out to draw evolutionary inferences about disease agents, unveil complex zoonotic cycles, assess vector competence, and discover genetic variations of pathogens. At the same time, research laboratories should develop diagnostic assays and protocols to roll out to public laboratories for surveillance purposes. However, while diagnostic tests exist for most pathogens, availability is often limited, especially in developing countries, and some kits are not in formats that can be deployed at the community level. Importantly, an effective disease outbreak communication strategy necessitates rapid and accurate data sharing among policy makers and collaborating partners to respond to public concerns and guide decisions on allocation of available resources.34
An infrastructure that supports a One Health mission for zoonotic disease investigations must encompass these diverse and complex interdisciplinary activities. In addition, because typical challenges faced in implementing a One Health platform have included effective coordination across stakeholder groups and the sustainability of government financial support,25 there must be mechanisms to promote interagency cooperation in the event of an outbreak (ie, ministries of health, agriculture, environment, and wildlife) and funding to acquire reagents and laboratory supplies for diagnostics and surveillance, as well as to meet other response needs. There must also be collaborations among manufacturing experts, biotechnologists, and regulatory agencies to enable production of safe and effective vaccines and therapeutics at a reasonable cost. Bringing vaccines and therapeutics to the public in turn will require vaccinology experts to partner with sociologists, industry, and governmental agencies to ensure compliance with vaccination programs by the target community.26
A Future of One Health
Despite lingering challenges, Uganda has experienced success in applying the One Health framework. The nongovernmental organization Conservation Through Public Health has successfully integrated pathogen surveillance into conservation and public health services in multiple districts throughout Uganda to monitor zoonotic disease transmissions among wildlife, humans, and livestock.35 Moreover, academic institutions have the capacity to train students and conduct interdisciplinary research, which not only builds a sustainable workforce but also enables collection of real-time data on which evidence-based policies can be based. For example, Makerere University has implemented a One Health Institute, a didactic training curriculum, and a Student One Health Innovation club to develop One Health competencies among students entering the workforce.36 Programs like that at Makerere University are instrumental for training collaborative-minded students who are bound for national and global health positions and committed to promoting a One Health approach. The viral ecology of bats in Uganda offers another unique One Health opportunity, given that Uganda has an infrastructure for infectious disease response and a history of viral hemorrhagic fever outbreaks, including of strains associated with bats. In our experience, interdisciplinary partnerships that span academic, private, and government entities bring together the necessary interdisciplinary expertise to carry out ecological and infectious disease research on bats that supports actionable policy and is built on the recognition that public health protection is not limited to human-focused efforts but is intimately connected with environmental health and conservation initiatives. Prevention of the next pandemic associated with a zoonotic disease agent may depend upon our taking a collaborative, One Health approach to public health protection.
References
- Plowright RK, Parrish CR, McCallum H, et al. Pathways to zoonotic spillover. Nat Rev Microbiol. 2017;15(8):502-510.
-
One Health basics. Centers for Disease Control and Prevention. Reviewed September 28, 2023. Accessed March 5, 2023. https://www.cdc.gov/onehealth/basics/index.html
-
Sorvillo TE, Rodriguez SE, Hudson P, et al. Towards a sustainable One Health approach to Crimean-Congo hemorrhagic fever prevention: focus areas and gaps in knowledge. Trop Med Infect Dis. 2020;5(3):113.
- Bird BH, Mazet JAK. Detection of emerging zoonotic pathogens: an integrated One Health approach. Annu Rev Anim Biosci. 2018;6(1):121-139.
- Anholt RM, Stephen C, Copes R. Strategies for collaboration in the interdisciplinary field of emerging zoonotic diseases. Zoonoses Public Health. 2012;59(4):229-240.
-
Mubareka S, Amuasi J, Banerjee A, et al. Strengthening a One Health approach to emerging zoonoses. Facets. 2023;8:1-64.
- Frick WF, Kingston T, Flanders J. A review of the major threats and challenges to global bat conservation. Ann N Y Acad Sci. 2020;1469(1):5-25.
- Rupprecht CE, Turmelle A, Kuzmin IV. A perspective on lyssavirus emergence and perpetuation. Curr Opin Virol. 2011;1(6):662-670.
- Aréchiga Ceballos N, Vázquez Morón S, Berciano JM, et al. Novel lyssavirus in bat, Spain. Emerg Infect Dis. 2013;19(5):793-795.
- Lau SKP, Woo PCY, Li KSM, et al. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci U S A. 2005;102(39):14040-14045.
-
Anthony SJ, Johnson CK, Greig DJ, et al; PREDICT Consortium. Global patterns in coronavirus diversity. Virus Evol. 2017;3(1):vex012.
-
Drexler JF, Corman VM, Müller MA, et al. Bats host major mammalian paramyxoviruses. Nat Commun. 2012;3:796.
- Albariño CG, Foltzer M, Towner JS, et al. Novel paramyxovirus associated with severe acute febrile disease, South Sudan and Uganda, 2012. Emerg Infect Dis. 2014;20(2):211-216.
-
Towner JS, Amman BR, Sealy TK, et al. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog. 2009;5(7):e1000536.
- Buttke DE, Decker DJ, Wild MA. The role of One Health in wildlife conservation: a challenge and opportunity. J Wildl Dis. 2015;51(1):1-8.
- Amman BR, Nyakarahuka L, McElroy AK, et al. Marburgvirus resurgence in Kitaka Mine bat population after extermination attempts, Uganda. Emerg Infect Dis. 2014;20(10):1761-1764.
-
Vincenot CE, Florens FBV, Kingston T. Can we protect island flying foxes? Science. 2017;355(6332):1368-1370.
-
Kading RC, Kingston T. Common ground: the foundation of interdisciplinary research on bat disease emergence. PLoS Biol. 2020;18(11):e3000947.
- Decker DJ, Siemer WF, Wild MA, et al. Communicating about zoonotic disease: strategic considerations for wildlife professionals. Wildl Soc Bull. 2011;35(2):112-119.
-
Pretorius M, Markotter W, Keith M. Assessing the extent of land-use change around important bat-inhabited caves. BMC Zool. 2021;6(1):31.
-
GBatNet (Global Union of Bat Diversity Networks). Accessed July 20, 2023. https://www.gbatnet.org/
-
Home. IUCN SSC Bat Specialist Group. Accessed July 20, 2023. https://www.iucnbsg.org/
- Gyles C. One Medicine, One Health, one world. Can Vet J. 2016;57(4):345-346.
-
Siya A, Mafigiri R, Migisha R, Kading RC. Uganda mountain community health system—perspectives and capacities towards emerging infectious disease surveillance. Int J Environ Res Public Health. 2021;18(16):8562.
- Buregyeya E, Atusingwize E, Nsamba P, et al. Operationalizing the One Health approach in Uganda: challenges and opportunities. J Epidemiol Glob Health. 2020;10(4):250-257.
-
Saylors K, Wolking DJ, Hagan E, et al; PREDICT Consortium. Socializing One Health: an innovative strategy to investigate social and behavioral risks of emerging viral threats. One Health Outlook. 2021;3(1):11.
-
Plumptre AJ, Baisero D, Belote RT, et al. Where might we find ecologically intact communities? Front For Glob Change. 2021;4:626635.
- Hassell JM, Begon M, Ward MJ, Fèvre EM. Urbanization and disease emergence: dynamics at the wildlife–livestock–human interface. Trends Ecol Evol. 2017;32(1):55-67.
-
Uganda. BIOPAMA Reference Information System. Accessed March 5, 2023. https://rris.biopama.org/country/ug
- Plowright RK, Field HE, Smith C, et al. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus). Proc Biol Sci. 2008;275(1636):861-869.
-
Uganda Parliament. Uganda Wildlife Act, 2019. Uganda Legal Information Institute. September 27, 2019. Accessed September 21, 2023. https://ulii.org/akn/ug/act/2019/17/eng@2019-09-27#part_VI__sec_35
- Sempala SD. Institute profile: the Uganda Virus Research Institute. Trends Microbiol. 2002;10(7):346-348.
-
Kyakuwa N, Kikaire B. About us. Uganda Virus Research Institute—Republic of Uganda. Accessed March 5, 2023. https://www.uvri.go.ug/about-us
- EisBrenner T, Tipples G, Kuschak T, Gilmour M. Laboratory Response Checklist for Infectious Disease Outbreaks—preparedness and response considerations for emerging threats. Can Commun Dis Rep. 2020;46(10):311-321.
-
One Health. Conservation Through Public Health. Accessed July 24, 2023. https://ctph.org/one-health-program/
-
Atusingwize E, Ndejjo R, Tumukunde G, et al. Application of One Health approach in training at Makerere University: experiences from the One Health Workforce Project in Uganda. One Health Outlook. 2020;2(1):23.