Abstract
Drug shortages are a persistent and serious problem in the United States, affecting patient care and health care costs. This article canvasses factors that contribute to drug shortages, such as manufacturing complexity, price, and quality inspection records. This article further proposes an early warning system and payment, contracting, and pricing innovations to mitigate drug shortages and offers data-driven recommendations to stakeholders looking to protect the supply of quality medicines.
Supply Chain Disruption
Drug shortages impede health care delivery in the United States, affecting patient care and increasing health care costs.1,2,3 While the immediate supply chain disruptions triggered by the COVID-19 pandemic are gradually resolving, the United States continues to face persistent drug shortages. In the first quarter of 2023, US active drug shortages were at their highest levels since 2014.4
Drug shortages trigger ethical considerations regarding equity of access, information transparency, and crisis management. How should clinicians decide which patients should have access to scarce drugs and treatment alternatives? How should drug shortage information be communicated to patients and the public in a manner that is transparent, accurate, and responsible? What are the criteria for evaluating patient demand under crisis conditions?
This article quantitatively evaluates the impact of manufacturing complexity, price, and quality inspection records on drug shortages in the United States using publicly available data from government websites and the subscription-based Medicine Supply Map. By quantifying the factors correlated with drug shortages, stakeholders can make data-driven decisions to protect the supply of quality medicines, ultimately minimizing the ethical dilemmas triggered by drug shortages. Based on our findings, we advocate for the implementation of 2 strategic solutions aimed at fortifying the resilience of pharmaceutical supply chains: (1) investment in an early warning system for drug shortages and (2) exploration of payment, contracting, and pricing innovations.
Defining Drug Shortages in US Markets
Hospital pharmacists receive information about drug supply through wholesalers, manufacturers, and other hospitals, as well as the American Society of Health-System Pharmacists (ASHP) and the US Food and Drug Administration (FDA) shortage websites.5,6 While both ASHP and FDA report information on drug shortages for US markets, the organizations differ in their definitions and regulatory implications.
The FDA’s drug shortage list, managed by FDA drug shortage staff in the Center for Drug Evaluation and Research,6 focuses on national-level shortages of medically necessary drugs and requires an economic evaluation of manufacturers’ ability to supply the market for a given drug product to determine if the drug product is in shortage. According to the FDA website, “We consider a drug to be in shortage when the total supply of all versions of a commercially available product cannot meet the current demand, and a registered alternative manufacturer will not meet the current and/or projected demands for the potentially medically necessary use(s) at the patient level.”7
ASHP defines a drug shortage more broadly as “a supply issue that affects how the pharmacy prepares or dispenses a drug product or influences patient care when prescribers must use an alternative agent.”8 Because ASHP does not require an economic determination of a national supply-demand imbalance for a drug to be included on the shortage list, as shown in Figure 1, there are more drug products on the ASHP shortage list than the FDA shortage list.
Figure 1. Comparison of 271 Drug Products on FDA and ASHP Drug Shortage Lists
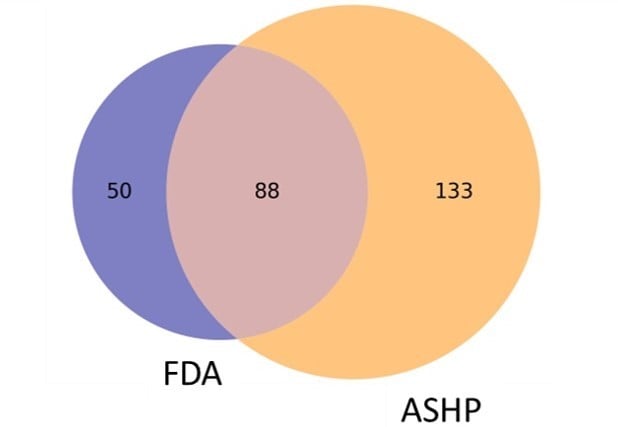
Data obtained from the US Food and Drug Administration5 and the American Society of Health-System Pharmacists6 on June 26, 2023.
Abbreviations: FDA, Food and Drug Administration; ASHP, American Society of Health-System Pharmacists.
The FDA’s drug shortage reporting, unlike ASHP’s, triggers certain regulatory actions. When a drug is placed on the FDA’s drug shortage list, pursuant to the Drug Quality and Security Act of 2013,9 certain regulatory restrictions are removed for 503B outsourcing facilities.10 Such facilities may produce compounded drugs that “are essentially copies of approved drugs.”10 Compounded drugs are made by pharmacists according to physicians’ prescriptions but are not FDA-approved and “pose a higher risk to patients … because they do not undergo the FDA’s premarket review for safety, effectiveness, or quality.”11 This increased risk to patients is balanced against availability during a drug shortage event. In addition to removing regulatory restrictions for 503B compounding, the FDA can expedite review of manufacturing proposals12 (eg, changes in manufacturing sites or active pharmaceutical ingredient [API] sourcing), extend the expiry date for certain drug products,13 and exercise temporary import exceptions to mitigate the impact of drug shortages (eg, the FDA’s temporary import approval for cisplatin from China with non-US labeling14).
Shortages’ Influence on Health Outcomes
Cost of labor. Hospital systems establish interdisciplinary teams to mitigate the impact of drug shortages.15 These drug shortage response teams are responsible for determining the organization’s supply forecast, evaluating alternative treatments, and changing patient care processes. Drug shortage response teams must also address ethical considerations related to patient access and patient disclosure, particularly in situations with few or no therapeutic alternatives. The administrative labor cost of managing US drug shortages was estimated to be $216 million per year in 20112 and $360 million per year in 2019.3
Cost of therapeutic alternatives. Changing therapeutic solutions to combat drug shortages creates numerous cost, safety, and efficacy concerns. A 2014 study estimated that drug shortages increased health care costs by roughly $209 million in 2013 due to patients switching to more expensive therapeutic alternatives.1 Changes to patient care processes, unusual dosing regimens, and worse adverse-effect profiles also increase the risk of medication errors for patients.16,17
Cost to patients and society. The true magnitude of drug shortages can only be measured through patient and societal impact. For example, the societal cost attributed to elevated mortality rates during the 2011 norepinephrine (a vasopressor used in treating septic shock) shortage has been estimated to be $13.7 billion.16 Because oncology drugs are inherently difficult to substitute due to their strict dosing regimens, often a shortage of a single drug product will drive supply risk for therapeutic alternative drug products, as in the case of cisplatin and carboplatin.18,19 As of June 26, 2023, 14 oncology drugs were in shortage, according to the FDA.6
Although a dollar amount cannot be placed on it, the risk of antimicrobial resistance (AMR) due to challenges to antibiotic supply, as exemplified by the recent amoxicillin shortage, also poses costs to patients and society.20 Effective antibiotic stewardship requires prescribers to issue “the right drug at the right dose at the right time for the right duration.”21 Switching to a more aggressive antibiotic tier based on supply constraints rather than patient need decreases the long-term efficacy of antibiotic drug products and poses a significant global health concern.6,22 Additionally, the prevalence of antibiotic drug shortages is intrinsically linked to an increased risk of use of substandard and falsified medicines.23 Poor-quality antibiotics increase the risk of AMR primarily through the administration of subtherapeutic dosing.24 Safeguarding the availability of quality antibiotics is thus critical to fighting AMR for patients globally.
Which Analytics Help Identify Drug Shortage Patterns?
Drug shortages stem from a complex interplay of economic, manufacturing, quality, and geographic factors. United States Pharmacopeia (USP) developed the Medicine Supply Map to identify, characterize, and quantify risks of disruption in the upstream pharmaceutical supply chain. USP identified 3 specific impacts of drug shortages, as defined by drugs’ inclusion on either the ASHP’s or FDA’s drug shortage list, at the drug product level.
Impact of dosage form on drug shortages. Injectable drug products are more complicated to manufacture than oral dosage forms. Compatibility studies, sterility assurance, and special handling considerations increase injectables’ manufacturing costs and risks of a negative quality event. These factors also increase the risk of a drug shortage. Figure 2 shows that, out of the 559 injectables tracked by the Medicine Supply Map, 178 (31.8%) were in shortage as of June 26, 2023. Rates of shortages are significantly lower for other dosage forms (see Figure 2).
Figure 2. Relative Shortage of 2476 Drug Products by Dosage Form
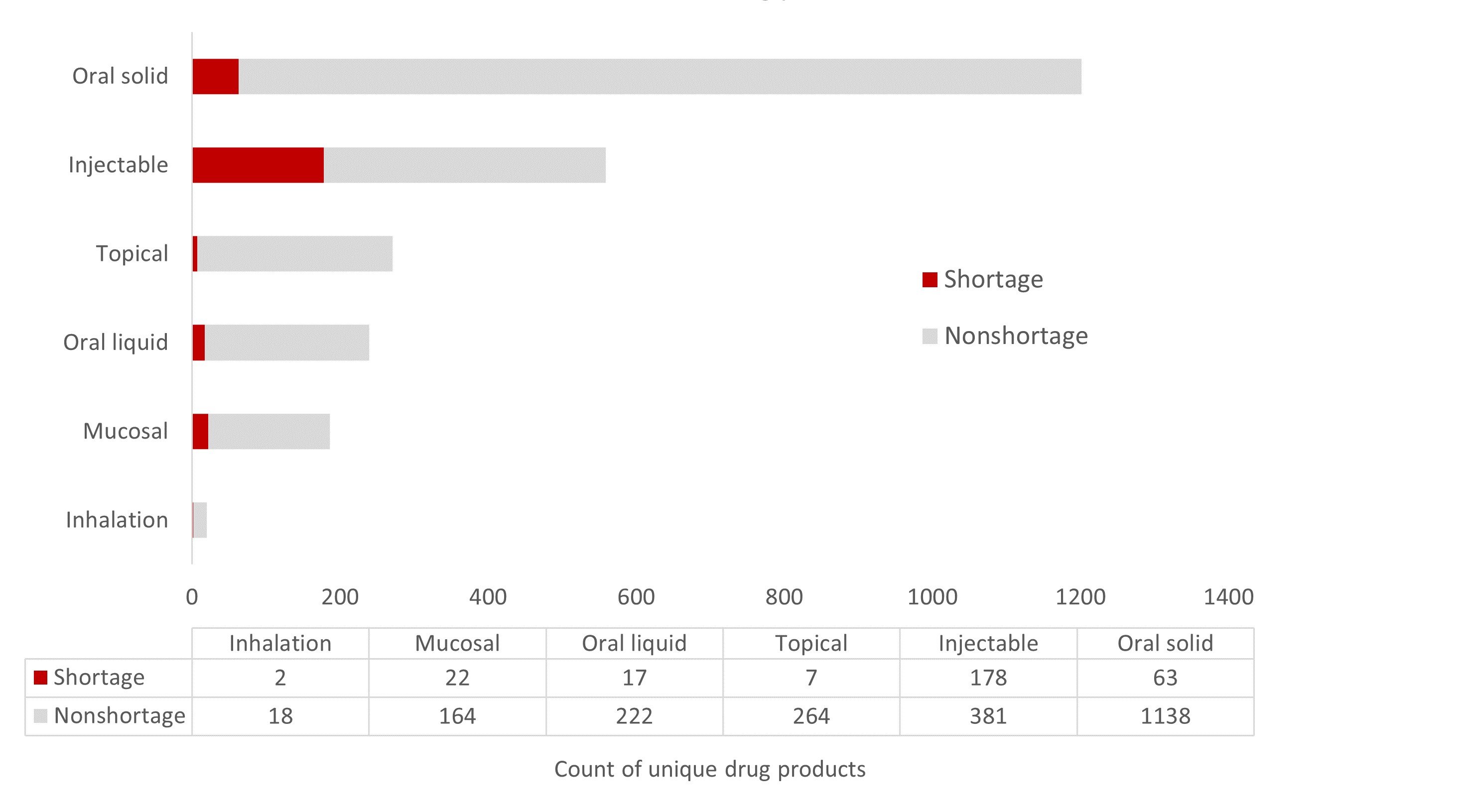
Data obtained from Medicine Supply Map on June 26, 2023.
Impact of drug product unit prices on injectable drug shortages. Lower-priced drugs experience more drug shortages, as manufacturers are less incentivized to invest in supply chain resilience. Figure 3 shows the relation between prices and drug product availability.
Figure 3. Impact of Unit Price on Supply Chain Vulnerability for Injectable Drug Products
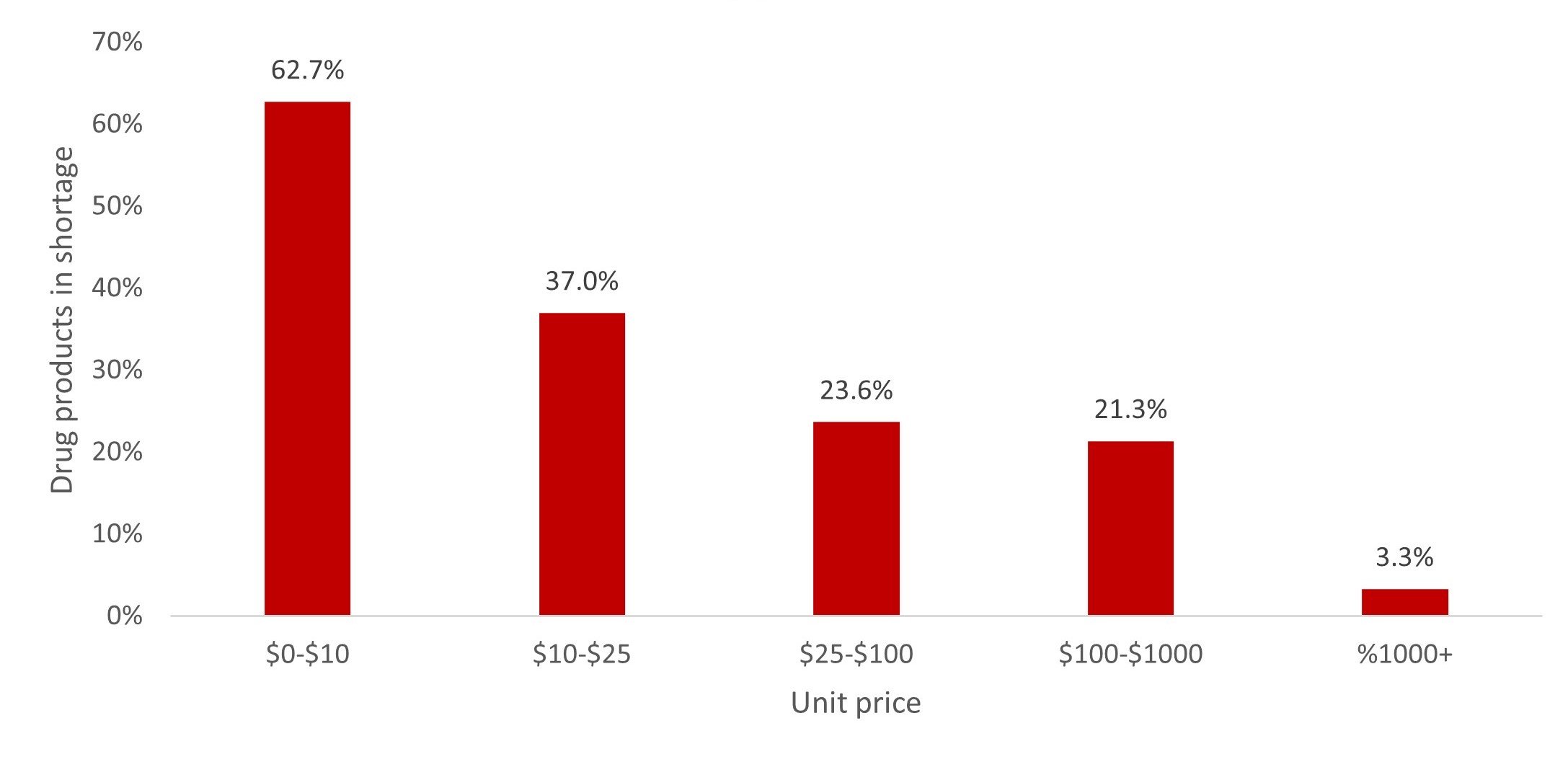
This analysis is based on publicly available pricing data from the US Department of Veterans Affairs25 and Medicare (Part B and Part D)26, 27on June 26, 2023.
Impact of manufacturing quality issues on drug shortages. Quality issues are correlated with drug shortages. According to the 2019 FDA report on drug shortages, “of 163 drugs that went into shortage between 2013 and 2017, 62 percent went into shortage after supply disruptions occurred that were associated with manufacturing or product quality problems.”28
Drug manufacturers are required to test products to ensure that medicines meet the quality specifications approved by the FDA or articulated in a USP quality monograph. The FDA undertook 2442 risk-based drug product and biologics inspections in 2022.29 FDA inspections serve an important function by helping to protect patients and foster manufacturing quality compliance. Medicines that do not meet quality requirements must be reported to the FDA and withheld from the market.
To quantify the impact of quality issues on drug shortages, the Medicine Supply Map examined the relation between FDA inspection outcomes and drug shortages. We analyzed the proportion of inspections resulting in an official action indicated (OAI) record. The OAI inspection outcome means regulatory or administrative actions are recommended to correct quality issues identified in a manufacturing facility.30 Figure 4 shows that drug products produced at facilities with a higher portion of OAI inspection records were more likely to be in shortage.
Figure 4. Impact of Manufacturing Quality Issues on Supply Chain Vulnerability
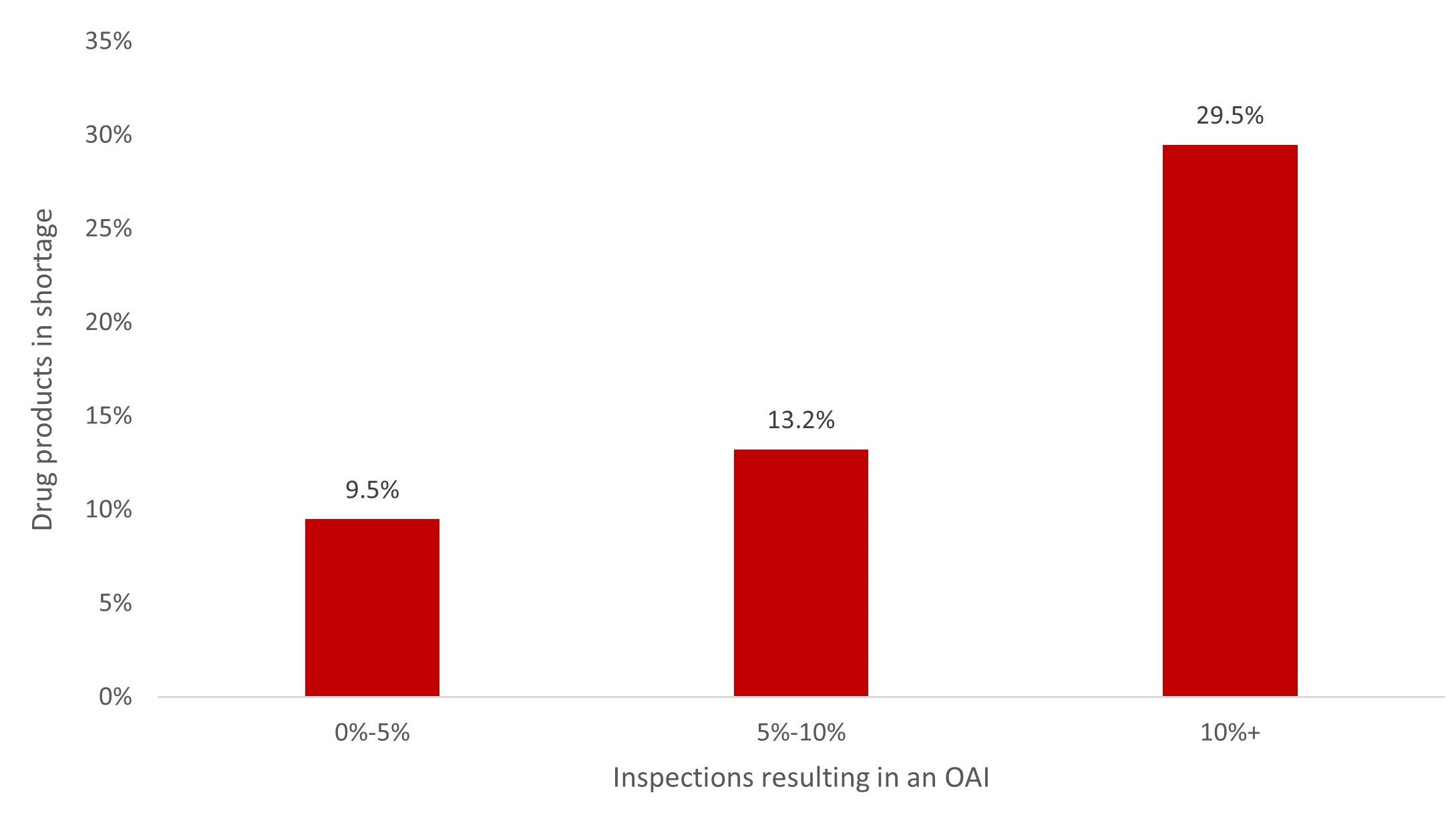
Data obtained from Medicine Supply Map on June 26, 2023.
Abbreviation: OAI, official action indicated.
Next Steps for Better Analytics and Sharper Surveillance
The journey of a molecule from an API to its final patient destination involves a network of specialized entities. API manufacturers create the primary therapeutic substance, drug product manufacturers transform the API into a consumable medication, distributors and wholesalers transport the drug products, and pharmacies help health care professionals administer medicines to patients. Because pharmaceutical supply chain data are heavily siloed within each specialized entity, solutions to drug shortages must bridge the information gaps across the supply chain. We propose the following solutions to strengthen patient access to quality medicines.
- Invest in an early warning system for drug shortages. Enhancing insight into drug shortages and quantifying the relation between drugs in shortage and explanatory variables can enable stakeholders to make more informed decisions about how to protect patient access to quality medicines. Early warning systems should extend beyond drug product and API manufacturing to key starting materials and excipients to provide a more comprehensive view of risks of disruption in the pharmaceutical supply chain landscape.
- Explore payment, contracting, and pricing innovations. Lower prices disincentivize manufacturers from investing in advanced quality systems and supply chain redundancy. A fundamental shift is needed in the market for lower-priced drugs to guarantee more certainty and predictability of both demand and supply and to increase the value placed on a drug’s supply chain resiliency and quality in addition to its price. Policy makers and public and private drug purchasers should explore the establishment and utilization of payment, contracting, and purchasing models that value and incentivize drug product quality and supply chain resilience.
Conclusion
The recent wave of new drug shortages provides the industry with an opportunity to rethink and reshape pharmaceutical supply chains and health care systems. We believe investments in an early warning analytics tool could help industry stakeholders make more informed supply chain decisions. A proactive, strategic, and collaborative approach that incorporates policy, industry, and health care stakeholders could help ensure a stable drug supply and promote better health outcomes for patients.
References
-
Premier. Drug shortages 2014: a Premier healthcare alliance update. Premier; 2014. Accessed November 16, 2023. https://op.bna.com/hl.nsf/r?Open=nwel-9gqqv6
- Kaakeh R, Sweet BV, Reilly C, et al. Impact of drug shortages on US health systems. Am J Health Syst Pharm. 2011;68(19):1811-1819.
-
Vizient. Drug shortages and labor costs: measuring the hidden cost of drug shortages in US hospitals. Vizient; 2019. Accessed November 15, 2023. https://wieck-vizient-production.s3.us-west-1.amazonaws.com/page-Brum/attachment/c9dba646f40b9b5def8032480ea51e1e85194129
-
Drug shortages statistics. American Society of Health-System Pharmacists. Accessed June 27, 2023. https://www.ashp.org/Drug-Shortages/Shortage-Resources/Drug-Shortages-Statistics
-
Current drug shortages. American Society of Health-System Pharmacists. Accessed June 27, 2023. https://www.ashp.org/Drug-Shortages/Current-Shortages
-
FDA drug shortages. US Food and Drug Administration. Accessed November 16, 2023. https://www.accessdata.fda.gov/scripts/drugshortages/default.cfm
-
Frequently asked questions about drug shortages. US Food and Drug Administration. Reviewed April 6, 2023. Accessed June 27, 2023. https://www.fda.gov/drugs/drug-shortages/frequently-asked-questions-about-drug-shortages
-
Drug shortages FAQs. American Society of Health-System Pharmacists. Accessed June 27, 2023. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-FAQs
-
Drug Quality and Security Act, Pub L No. 113-54, 127 Stat 587 (2013).
-
Center for Drug Evaluation and Research. Compounded drug products that are essentially copies of approved drug products under Section 503B of the federal Food, Drug, and Cosmetic Act: guidance for industry. US Food and Drug Administration; 2018. Accessed November 22, 2023. https://www.fda.gov/media/98964/download
-
Drug compounding and drug shortages. US Food and Drug Administration. Reviewed March 24, 2023. Accessed June 27, 2023. https://www.fda.gov/drugs/human-drug-compounding/drug-compounding-and-drug-shortages
-
Thakur E; Center for Drug Evaluation and Research. FDA drug topics: drug shortages: root causes and potential solutions. US Food and Drug Administration. Accessed October 30, 2023. https://www.fda.gov/media/159480/download
-
Search list of extended use dates to assist with drug shortages. US Food and Drug Administration. Reviewed May 4, 2023. Accessed June 27, 2023. https://www.fda.gov/drugs/drug-shortages/search-list-extended-use-dates-assist-drug-shortages
-
Xunliao Y; Qilo Pharmaceutical. Temporary importation of CISplatin injection with non-US labeling to address drug shortage. US Food and Drug Administration. May 24, 2023. Accessed October 30, 2023. https://www.fda.gov/media/169001/download
-
How hospital and health-system pharmacists manage drug shortages. American Society of Health-System Pharmacists. Accessed October 30, 2023. https://www.ashp.org/-/media/assets/drug-shortages/docs/ASHP-drug-shortages-infographic.pdf
-
Wosińska ME, Frank RG. Federal policies to address persistent generic drug shortages. Hamilton Project; 2023. Accessed June 27, 2023. https://www.brookings.edu/wp-content/uploads/2023/06/20230621_ES_THP_GSI_Report_Final.pdf
- Hughes KM, Goswami ES, Morris JL. Impact of a drug shortage on medication errors and clinical outcomes in the pediatric intensive care unit. J Pediatr Pharmacol Ther. 2015;20(6):453-461.
-
Weintraub K. A short supply of cancer drugs has doctors and patients worried: “we’re at a critical juncture.” USA Today. May 30, 2023. Accessed August 17, 2023. https://www.usatoday.com/story/news/health/2023/05/30/cancer-drug-chemotherapy-shortage-solutions-explained/70253377007/
-
Oliveira L, Caquito JM Jr, Rocha MS. Carboplatin as an alternative to cisplatin in chemotherapies: new insights at single molecule level. Biophys Chem. 2018;241:8-14.
-
Swetlitz I. Amoxicillin shortage reveals fragility of antibiotic supply. Bloomberg. November 2, 2022. Accessed June 27, 2023. https://www.bloomberg.com/news/newsletters/2022-11-02/amoxicillin-shortage-reveals-fragility-of-antibiotic-supply
-
Hyun D, Zetts R. How one urgent care doctor fights antibiotic resistance. Pew Charitable Trusts. November 19, 2019. Accessed June 27, 2023. https://pew.org/35i01FK
-
Murray CJL, Ikuta KS, Sharara F, et al; Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629-655.
-
Raghavendran V, Christian M. Supply chain vulnerabilities exist for antimicrobial medicines: USP medicine supply map analysis. United States Pharmacopeia. May 24, 2022. Accessed June 27, 2023. https://qualitymatters.usp.org/index.php/supply-chain-vulnerabilities-for-antimicrobial-medicines
- Andersson DI, Hughes D. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol. 2014;12(7):465-478.
-
Office of Procurement, Acquisition and Logistics (OPAL): pharmaceutical prices. US Department of Veterans Affairs. Updated November 19, 2023. Accessed November 16, 2023. https://www.va.gov/opal/nac/fss/pharmprices.asp
-
ASP pricing files. Centers for Medicare and Medicaid Services. Accessed October 26, 2023. https://www.cms.gov/medicare/payment/all-fee-service-providers/medicare-part-b-drug-average-sales-price/asp-pricing-files
-
Prescription drug plan formulary, pharmacy network, and pricing information files for order. Centers for Medicare and Medicaid Services. Updated September 6, 2023. Accessed October 26, 2023. https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/NonIdentifiableDataFiles/PrescriptionDrugPlanFormularyPharmacyNetworkandPricingInformationFiles
-
Drug Shortages Task Force. Drug Shortages: Root Causes and Potential Solutions. US Food and Drug Administration; 2019. Updated February 21, 2020. Accessed October 30, 2023. https://www.fda.gov/media/131130/download
-
Office of Planning, Evaluation, and Risk Management. Annual report on inspections of establishments CY 2022. US Food and Drug Administration; 2022. Accessed October 30, 2023. https://www.fda.gov/media/167520/download
-
Inspection classifications. US Food and Drug Administration. Reviewed May 15, 2023. Accessed June 27, 2023. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-basics/inspection-classifications



