Abstract
Zoonoses are infectious diseases that pass from an animal to a human. Of all emerging pathogens studied, zoonoses are the majority. As was seen with SARS-CoV-2, zoonoses can cause enormous morbidity and mortality around the world. Curbing these infections is of great interest, but combatting infections that start in various animal species comes with a multitude of challenges.
Case
Dr Z is a zoonotic infectious diseases specialist giving a talk as part of a panel session at a conference about emerging epidemic and pandemic disease transmission from nonhuman to human animals. Dr Z explains that some microbes do not cause disease in livestock or wild nonhuman animals but that nonhuman animal pathogens can “spill over” to humans. Dr Z offers examples: Nipah virus from fruit bats spreads to livestock and humans, Middle East Respiratory Syndrome (MERS) CoV spreads from camels to humans, and Crimean-Congo hemorrhagic fever virus is carried by ticks to livestock and humans. Dr Z then describes how vaccinating both domestic and wild nonhuman animals can mitigate much of the transmission to humans.
Commentary
Of all emerging pathogens studied, about 75% are due to zoonosis.1 The United States Agency for International Development (USAID) project PREDICT, run by the UC Davis One Health Institute, has discovered over 900 novel viruses in its surveillance around the world.2 While not all of these viruses can jump from nonhuman to human animals, some certainly do. Controlling zoonotic threats from livestock and companion animals is not new. Dr Z can elaborate on several ways to protect the human population. Controlling vectors of disease transmission can be as straightforward as eliminating standing water where mosquitos breed or as complex as genetically modifying flying insects. Animal inoculation, another method of control, is an ancient practice that opened the door to the science of vaccination in both animals and humans. However, a more novel approach is to vaccinate individuals of the species that transmit the disease.
Insecticides and Genetic Modification
Dr A could point out that we already mitigate the risk of zoonoses through nonvaccine prevention methods. Mosquito control programs, such as using insecticides, are old and often effective temporarily. With time, however, resistance to insecticides can occur,3 and these programs require vigilance to keep the population in check. Genetic modification of mosquitos—specifically, Aedes aegypti mosquitoes that transmit Dengue and Zika viruses, among others—is also effective,4 although not without controversy.5 These genetically modified mosquitoes are released into the wild, where they can lay eggs carrying the same modified genes, leading to death of the mosquito before adulthood. With time, the population of this specific mosquito species (but not others) will decline but not be eliminated. If the genetically modified mosquitos are not continually released year after year, the population will return to “normal levels.” Effective mosquito control programs thus incorporate multiple modalities, such as a combination of irradiation, larvicides, and monitoring standing water, to have the largest impact.6
Animal Vaccination
Dr A could note that though vaccinating humans helps reduce morbidity and mortality in people, it does nothing to eliminate the threat of disease. To do that, we must inoculate the animal population that the pathogen first infects. If we can rid the primary host of the infection, then we will prevent future disease in that species and in secondary species, such as ours.
Dr Z could explain that protecting livestock via vaccination protects humans in a variety of ways. We vaccinate household pets for many diseases, such as rabies and leptospirosis, which can infect both animals and humans.7 Vaccinating livestock helps prevent foodborne diseases, such as cysticercosis (from Taenia solium) and toxoplasmosis (from Toxoplasma gondii), which can devastate livestock populations as well as cause human infections. According to Sander et al, “It is generally accepted that the administration of vaccines for foodborne infections is the best-available public health intervention” not only for improving the overall health and reducing the mortality of both animals and humans, but also for promoting socioeconomic development in communities that rely on livestock for their livelihood.8
Nevertheless, animal vaccines are not without some risks. For example, there have been reports of accidental human inoculation with Brucella vaccines developed for cows and sheep.9 The Centers for Disease Control and Prevention tracks these cases and has information for post-exposure prophylaxis for veterinarians or others accidentally exposed.10
Moreover, these traditional vaccines are of limited value. While we often think of vaccines in an injectable form for one person, scientists have developed different vaccine types that promote self-dissemination in animals. For example, putting a vaccine on a bat’s fur is an example of a transferable vaccine. That bat may encounter a dozen other bats, thereby transferring the vaccine through typical colony grooming behaviors. However, the vaccination effort stops there. The newly vaccinated bats do not have any vaccine on their fur, so they will not transfer any vaccine to others. Transmissible vaccines require an initial injection, but then that animal can transmit the vaccine to others via typical social interactions.11,12 The transmissibility of the vaccine will determine how long it persists within the species. If the basic reproductive number, the R0, of the vaccine, is low, then the vaccinated animal might not actually pass it along to another animal of the same species. The theoretical benefit of transmissible vaccines is that vaccines with a high R0 will have an extensive horizontal spread.13 Those secondarily vaccinated animals can then transmit the vaccine when they interact with other animals. While transmissible vaccines might cause a faster rise in the number of vaccinated animals, unintended consequences, if any, will persist in the population as well. Dr A must report that both types of self-disseminating vaccines require expert knowledge of the pathogen target, the reservoir host immune system and behaviors, and possible consequences of the vaccination efforts.14
Protecting the Most Vulnerable
Most zoonoses are considered tropical diseases, and many fall into the category of neglected tropical diseases (NTD),15 which are often due to poor sanitation and crowded living conditions, exacerbated by personal poverty and a lack of protective systems in the country. NTDs are ancient diseases that have been afflicting the poorest on this planet for centuries. Another hallmark of NTDs is that they cause chronic, debilitating symptoms and have a relatively low mortality rate.16 As a result, people live with disfigurement, become unable to work, and are further subjected to poverty and stigmatization. In many areas, children are infected with parasites like hookworm and become anemic and malnourished,16 making it difficult to learn (if schooling is an option) and difficult to work and earn a wage.15
NTDs mainly affect people in Asia, Africa, and Latin America (see Figure). As such, preventing transmission of NTDs is also a matter of equity and social justice that cannot be ignored. These areas and diseases are all but forgotten in the high-income countries where vaccine development occurs. Because there is no traditional commercial market for vaccines for infections like NTDs, vaccine development has been slow or nonexistent. There have also been technical difficulties in finding human vaccines for these diseases.16
Figure. Geographic Overlap of the Neglected Tropical Diseasesa
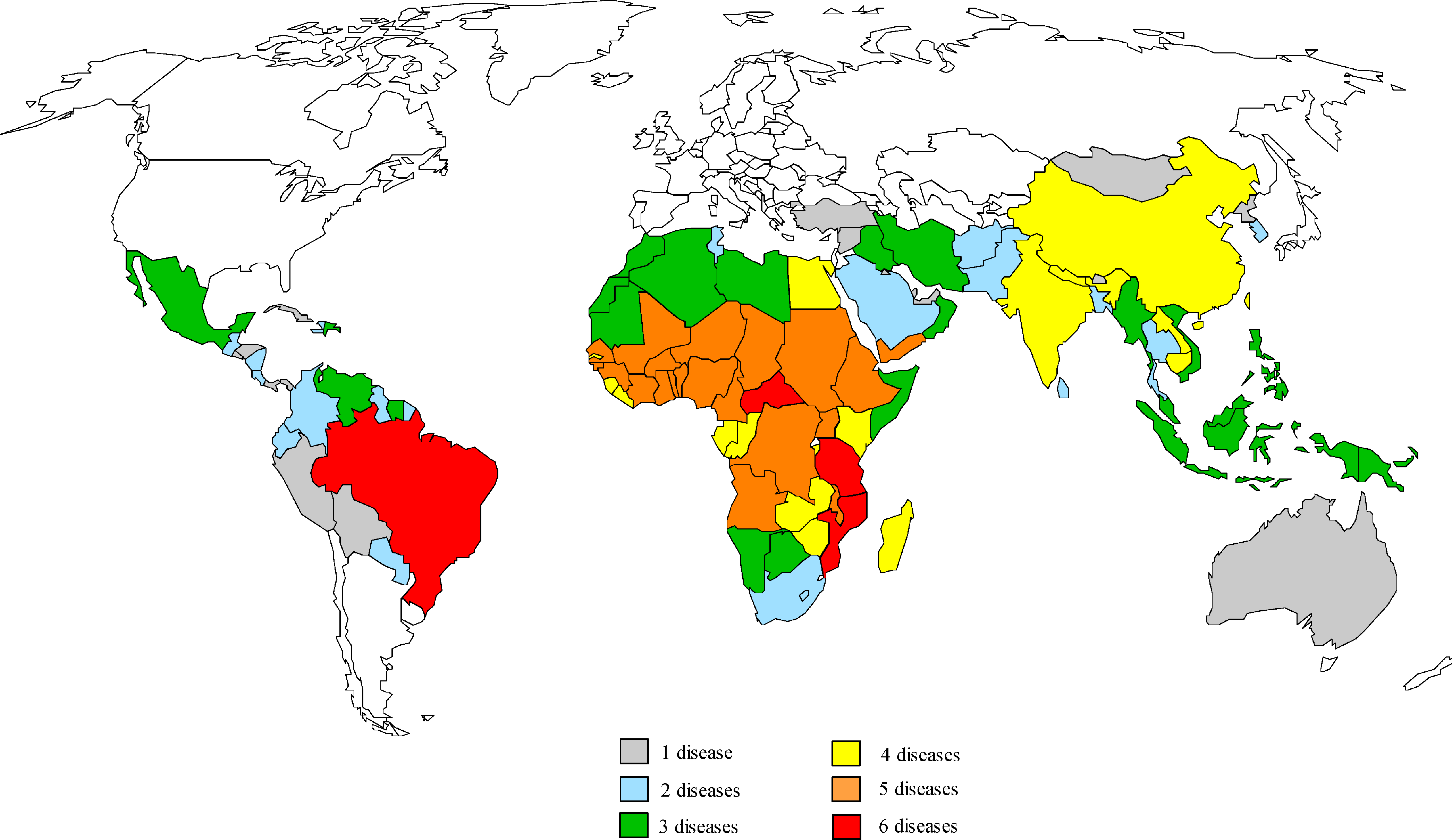
Reproduced from Molyneux DH, Hotez PJ, Fenwick A.17 © 2005 Molyneux et al. Licensed under Creative Commons Attribution 4.0 International.
a Map prepared by Molly Brady, Emory University.
High-income countries, Dr A can point out, must realize that the risk of a tropical disease in their lands is not as far off as they might think. There have been recent cases of “homegrown” malaria in the United States,18 after mosquitoes native to the United States bit people who returned with malaria after a trip abroad. These mosquitoes could then bite and infect the traveler’s contacts, perpetuating the infection cycle when the contacts in turn get bitten by mosquitoes. Similarly, there was a case of Dengue virus infection in the United States of a person who lived near someone who had returned with the virus after traveling to an endemic region.19
As we have seen during the COVID-19 pandemic, improving public health structures in developing countries benefits everyone. While the rapid development of the SARS-CoV-2 vaccine, which relied heavily on previous vaccines for other coronaviruses, has raised hopes for development of vaccines for NTDs, companies are not incentivized to develop vaccines for persons who cannot pay for them. Restructuring this financial model is essential but will take time. Other options must be explored.
Where to invest our research efforts remains unclear. Working on human vaccines would have some advantages, as most NTDs have a clear geography. However, many populations suffer from the risk of more than one NTD (see Figure), so human vaccines would need to offer protection for multiple diseases (eg, Tdap vaccines offer protection against tetanus, diphtheria, and pertussis with one shot) to maximize the reach of a vaccine campaign. The implementation of any mass vaccination campaign would require Herculean investment of financial, human, and educational capital. While challenges of developing animal vaccines are clearly different, they also would require significant investment. Wealthier countries must recognize that it is in their self-interest to tackle these diseases now rather than risk outbreaks in the future.
Summary
Zoonoses remain a major global threat. Reacting to a pandemic, as we saw with COVID-19, is costly both in financial terms as well as in human lives lost. It is essential to be proactive, lest we lose another almost 7 million people in the next pandemic.20 Carpenter et al note: “The potential for zoonotic diseases to affect human, animal, plant, and environmental health, global food security, and economic stability highlights the need for effective interventions that target prevention at multiple levels.”21 Human and nonhuman animal vaccines are very promising avenues for achieving this goal.
References
- Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356(1411):983-989.
-
One Health Institute. PREDICT. School of Veterinary Medicine, University of California, Davis. Accessed June 20, 2023. https://ohi.vetmed.ucdavis.edu/programs-projects/predict-project
- Brogdon WG, McAllister JC. Insecticide resistance and vector control. Emerg Infect Dis. 1998;4(4):605-613.
-
Genetically modified mosquitoes. Centers for Disease Control and Prevention. Reviewed July 25, 2022. Accessed June 29, 2023. https://www.cdc.gov/mosquitoes/mosquito-control/community/emerging-methods/genetically-modified-mosquitoes.html
-
Cisnetto V, Barlow J. The development of complex and controversial innovations. Genetically modified mosquitoes for malaria eradication. Res Policy. 2020;49(3):103917.
-
Mosquito control in a community. Centers for Disease Control and Prevention. Reviewed July 25, 2022. Accessed June 29, 2023. https://www.cdc.gov/mosquitoes/mosquito-control/community/index.html
-
Vaccinations. American Veterinary Medical Association. Accessed November 1, 2023. https://www.avma.org/resources-tools/pet-owners/petcare/vaccinations
-
Sander VA, Sánchez López EF, Mendoza Morales L, Ramos Duarte VA, Corigliano MG, Clemente M. Use of veterinary vaccines for livestock as a strategy to control foodborne parasitic diseases. Front Cell Infect Microbiol. 2020;10:288.
- Ashford DA, di Pietra J, Lingappa J, et al. Adverse events in humans associated with accidental exposure to the livestock brucellosis vaccine RB51. Vaccine. 2004;22(25-26):3435-3439.
-
Exposure to EB51: how to reduce risk of infection. Centers for Disease Control and Prevention. Reviewed August 31, 2017. Accessed June 9, 2023. https://www.cdc.gov/brucellosis/veterinarians/rb51-reduce-risk.html
- Bárcena J, Morales M, Vázquez B, et al. Horizontal transmissible protection against myxomatosis and rabbit hemorrhagic disease by using a recombinant myxoma virus. J Virol. 2000;74(3):1114-1123.
- Murphy AA, Redwood AJ, Jarvis MA. Self-disseminating vaccines for emerging infectious diseases. Expert Rev Vaccines. 2016;15(1):31-39.
- Bull JJ, Smithson MW, Nuismer SL. Transmissible viral vaccines. Trends Microbiol. 2018;26(1):6-15.
- Nuismer SL, Bull JJ. Self-disseminating vaccines to suppress zoonoses. Nat Ecol Evol. 2020;4(9):1168-1173.
-
Neglected tropical diseases. Centers for Disease Control and Prevention. Reviewed June 19, 2023. Accessed June 29, 2023. https://www.cdc.gov/globalhealth/ntd/index.html
- Bethony JM, Cole RN, Guo X, et al. Vaccines to combat the neglected tropical diseases. Immunol Rev. 2011;239(1):237-270.
-
Molyneux DH, Hotez PJ, Fenwick A. “Rapid-impact interventions”: how a policy of integrated control for Africa’s neglected tropical diseases could benefit the poor. PLoS Med. 2005;2(11):e336.
-
Tropical diseases. American Society of Tropical Medicine and Hygiene. Accessed June 29, 2023. https://www.astmh.org/education-resources/tropical-medicine-q-a/major-tropical-diseases
- Kretschmer M, Collins J, Dale AP, et al. Notes from the field: first evidence of locally acquired Dengue virus infection—Maricopa County, Arizona, November 2022. MMWR Morb Mortal Wkly Rep. 2023;72(11):290-291.
-
WHO Coronavirus (COVID-19) Dashboard. World Health Organization. Updated October 18, 2023. Accessed June 9, 2023. https://covid19.who.int/
-
Carpenter A, Waltenburg MA, Hall A, et al; The Vaccine Preventable Zoonotic Disease Working Group. Vaccine preventable zoonotic diseases: challenges and opportunities for public health progress. Vaccines (Basel). 2022;10(7):993.



