Abstract
Use of body mass index (BMI) as a health care metric is controversial, especially in candidacy assessments for gender-affirming surgery. When considering experiences of fat trans individuals, it is important to advocate for equitable divisions of responsibility for and recognition of systemic fat phobia. This commentary on a case suggests strategies for increasing equitable access to safe surgery for all body types. If surgeons use BMI thresholds, simultaneous effort must be made to advocate for data collection so that surgical candidacy criteria are evidence-based and equitably applied.
Case
ZZ is a trans man and a patient of Dr S, a surgeon at a clinic offering gender-affirming services, including hormone therapy, chest surgeries, and genital surgeries. During 5 years of hormone treatment, ZZ’s weight increased to a point at which he now has a BMI of 35, which is clinically considered class II obesity.1 As a result, he does not qualify for most gender-affirming surgeries (GAS) offered by Dr S at the clinic. ZZ is distressed and asks, “What was the point of hormone therapy if all it did was make me so fat I can’t get surgery?”
Dr S considers how to respond.
Commentary
Transgender, nonbinary, and other non-cisgender (henceforth referred to as trans) individuals with a body mass index (BMI) of at least 30 (referred to clinically as “obesity”),1 could be denied access to GAS2 due to systemic bias and social inequity. High BMI is associated with conditions such as sleep apnea,3,4,5 type 2 diabetes, gallbladder disease, and certain types of cancers.6 It is also associated with perioperative issues, including surgical site infection,7 increased operative time,8 and greater technical difficulty when operating9,10 and hence is often a primary factor in GAS candidacy.9 However, this risk metric can obscure other multifactorial causes11,12,13 that contribute to poor surgical outcomes.7,8,9,14 Moreover, some consider BMI thresholds to be a manifestation of weight stigma,9,11 or the negative stereotyping of and discrimination against fat individuals.12,15 (We use the word fat here as a neutral descriptor of body size in alignment with fat activists to help destigmatize the word.16)Weight stigma, among other biases, can cause clinicians to erroneously attribute a patient’s health issues to their body size.12 As a result of weight stigma, fat patients may be inclined to avoid clinical care.12
Equitable treatment requires that we consider the current surgical risks for fat patients, how weight stigma contributes to these risks,12 and the appropriate uses of BMI in clinical care. It is also important to acknowledge the problematic history of BMI, including the lack of validation for its use in non-cisgender populations of color11 and the relationship between weight stigma and racism.17,18 For example, among Black men, experience of major discrimination is associated with obesity.19
Here, we discuss weight stigma, inadequate empirical evidence of GAS risks associated with BMI, and how to reduce barriers to GAS for fat trans people like ZZ by addressing structural oppression. In the absence of definitive evidence of a direct causal link between high BMI and poor GAS outcomes, we propose a more holistic approach to surgical candidacy that includes shared decision making, wherein BMI is not used as the sole determinant of GAS access but is considered alongside weight stigma and factors like procedure type and body composition.
Weight Stigma
ZZ’s weight and his perception of how it affects his surgery access is mediated by internalized weight stigma. Internalized weight stigma poses concrete risks to patients by negatively influencing eating and exercise behavior,20 and it is also associated with depression and body shame.20 Even if ZZ ends up having surgery, he may struggle to find peer support, the benefits of which for surgery and medical care have been described in the existing literature for fat trans people who do not have easy access to GAS.20,21
Fat patients may also experience a lower quality of care due to clinician biases.12 Surgical teams can limit reinforcing these biases in clinical environments by questioning their own anti-fat attitudes, as well as by educating clinicians and staff members on the complexity of weight and weight change.12 Quality of care can be improved by using motivational interviewing and patient-centered communication12 and by shifting the focus from weight loss to the benefits of behavior changes, such as increased physical activity.12
Known and Anticipated Risks and Benefits
The benefits of GAS, including decreased gender incongruence, improved quality of life, and decreased suicide risk, cannot be understated.11,21 These data help make the case for proceeding with surgery despite potential risks associated with an elevated BMI.
Existing data on GAS indicate that the risk of complications is contingent on multiple factors, including procedure type, BMI, and body composition. Two studies have reported that gender-affirming mastectomy for patients with a BMI of 30 to 39.9 is relatively safe.22,23 Data on complication risk specific to genital GAS, however, is lacking9,11 and, with few exceptions,24 is not available for those above a BMI of 30 (see Tables 1 and 2).
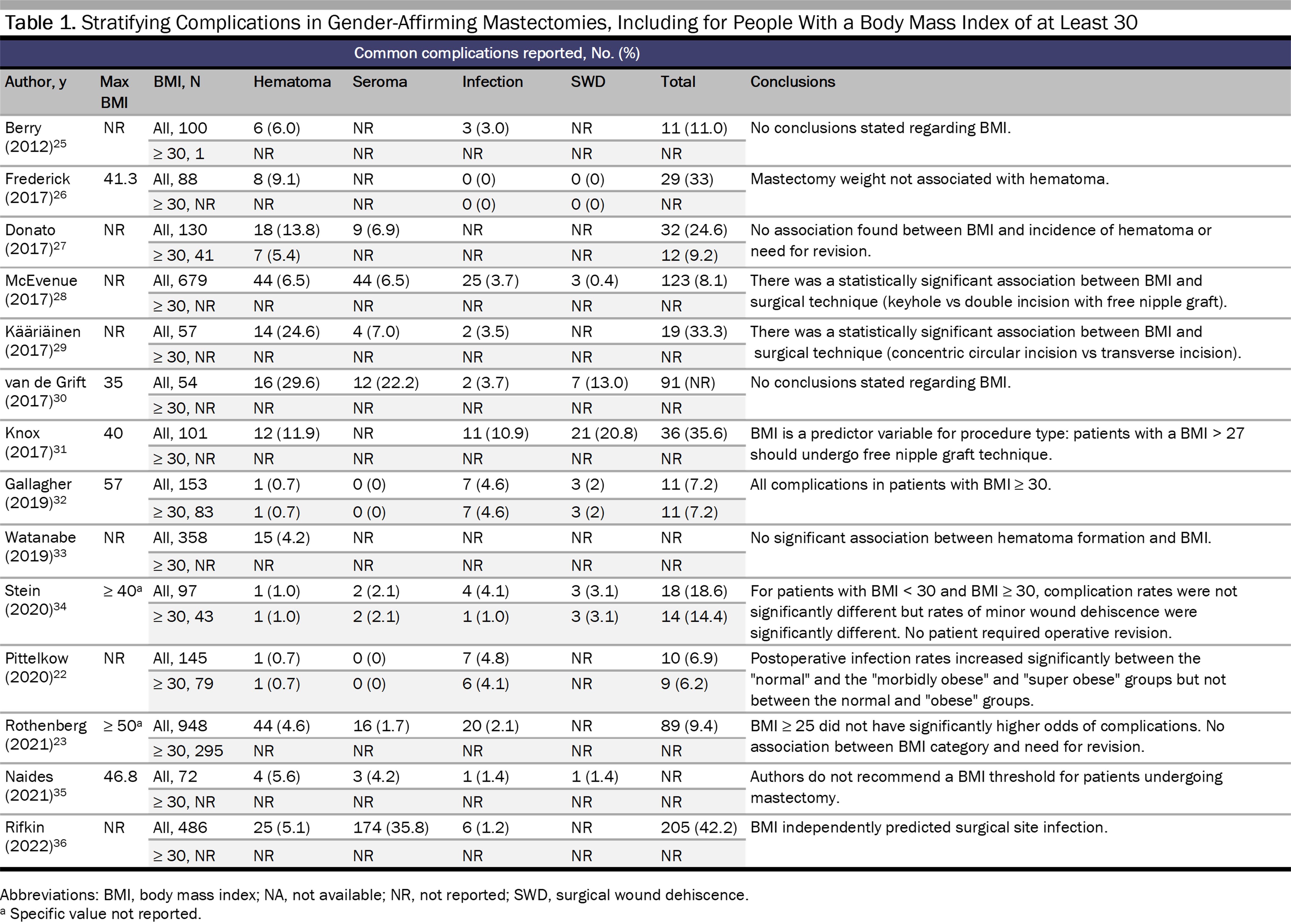
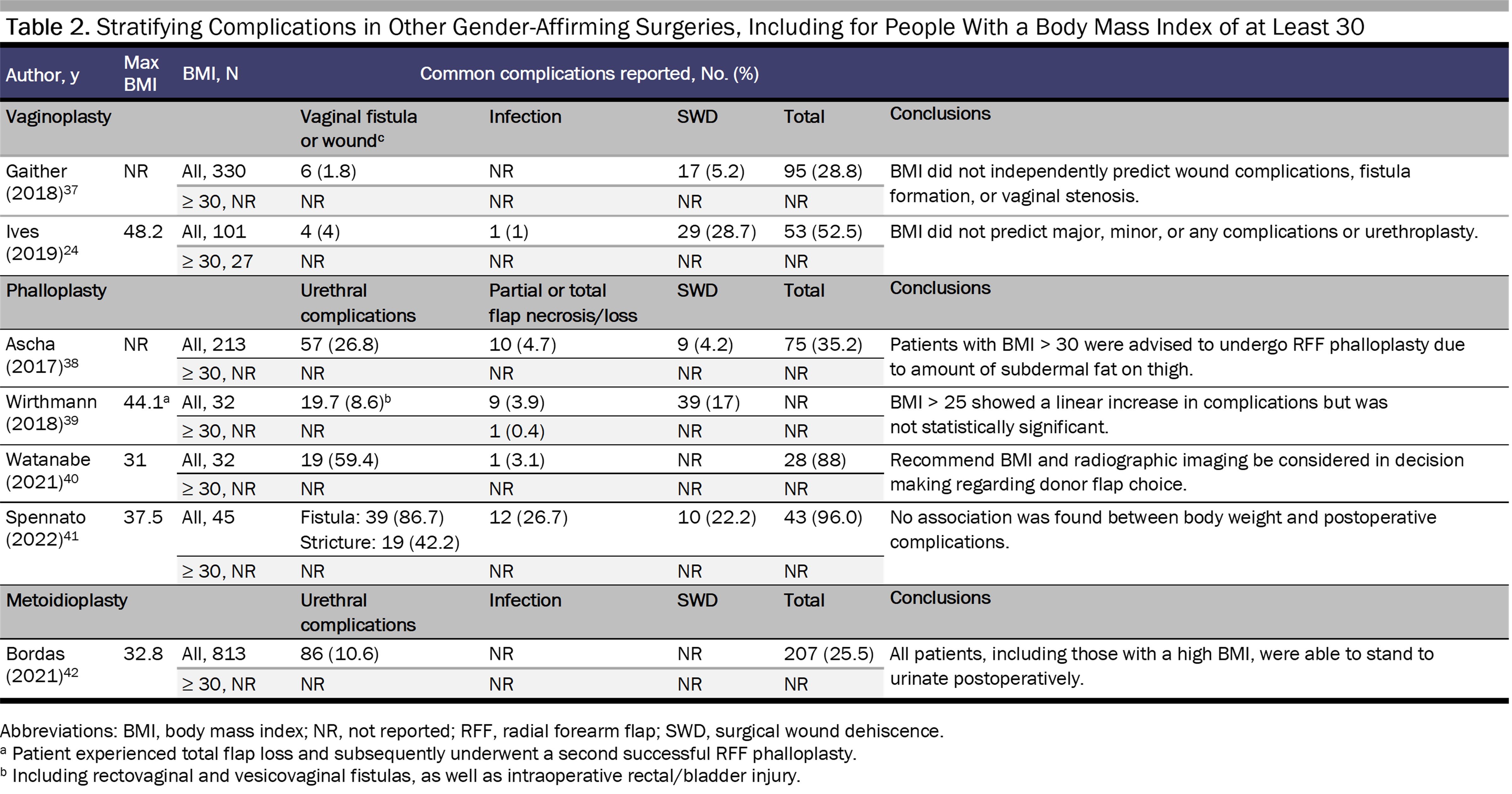
In this data’s absence we can extrapolate from similar procedures.9 For example, in robot-assisted laparoscopic radical prostatectomy, BMI correlates with pelvic visceral fat volume, pelvic width, and working space.43 More pelvic visceral fat can increase operative time and incidence of complications.43 However, in colorectal surgery, BMI appears to be less accurate at predicting the amount of visceral fat.44 Overall body composition therefore may be more helpful when estimating surgical risk, as BMI does not account for the effects of body composition on surgical outcomes. Reporting on surgical complications is also not standardized (see Tables 1 and 2).
Complications stratified by BMI provide more specific information on potential risks and outcomes, although only the studies by Stein et al34 and Gallagher et al32 analyze the data in this way. For gender-affirming mastectomy, a BMI of 30 or more is associated with hematoma,22,23,32,34 seroma,22,34,45 infection,22,32,34,36 and wound dehiscence.32,34,36 For phalloplasty, one study found no statistically significant relationship between a BMI of at least 25 and increased complications,25 although results may vary with type of reconstruction. In another study, one patient with a BMI of 44.1 experienced total flap loss and underwent a second successful phalloplasty, although this patient engaged in heavy smoking,39 a known risk factor for impaired wound healing independent of BMI.46 Ascha et al found that patients undergoing radial forearm flap phalloplasty experienced fewer complications and had a higher BMI than patients undergoing anterolateral thigh flap phalloplasty.38 However, Wirthmann et al showed that there was a trend (though not significant) toward complications for patients with a BMI greater than 25 undergoing radial forearm flap phalloplasty.39 Watanabe et al suggested that BMI can be useful, in tandem with radiographic imaging, when selecting type of donor flap to use for penile creation in phalloplasty.33 Similarly, for vaginoplasty, data on complications stratified by BMI are limited,24 and the existing data are too sparse to lead to definitive conclusions about the use of BMI in assessing surgical candidacy.
Risks for patients with a higher BMI precede the operating table, such as the risks accompanying weight loss attempts to qualify for surgery. Losing weight safely or sustainably is difficult and often not achievable for most patients recommended to pursue weight loss.47 It can even be harmful for some individuals to attempt any weight changes, especially those with an active or previous eating disorder, which is characteristic of a large portion of trans individuals.48,49 Additionally, permanent weight loss attempts often result in cycles of weight loss and regain, which are ineffective and have their own health risks.50
We must consider the ethics of recommending that patients pursue medical or surgical interventions for weight loss before undergoing GAS without evidence that weight loss will significantly affect surgical outcomes as well as long-term outcomes in cases in which patients lose weight preoperatively and then experience postoperative weight regain. Lastly, some fat individuals regard their body size as part of their identity16,51 and do not want to attempt any kind of weight change. Recommending weight loss to fat individuals whose trans identities incur significant social criticism can similarly be perceived as a negative judgment and thereby damage the patient-surgeon relationship.
Shared Decision Making
In the absence of ample data, shared decision making supports informed consent. Increased risk of complications is often used to rationalize denying surgery to fat patients, as surgeons operating on individuals who may be at higher risk of complications could be accused of poor judgment or even face litigation if problems arise intraoperatively.52,53 Creating a more equitable division of decision-making responsibility between patients and surgeons can mitigate surgeons’ fears of performing unsafe surgeries or patients’ fears of experiencing poor outcomes. This goal can be achieved by proper informed consent through patient education and by allowing patients to be involved in the final decision.
Fear of litigation does not adequately justify refusal to operate, especially if the complications are manageable through wound care or revision surgeries. Even data on serious complications, like total flap loss, can help set patient expectations and possibly reduce legal action stemming from miscommunication. A paradigm of robust informed consent and collaboration encourages patient autonomy and strengthens patient-surgeon relationships.
Equitable Access to Surgery
Evidence regarding causes of fatness increasingly points toward macro-structural factors,54 which, alongside structural stigma, contribute to health inequalities.55,56 In the case of other stigmatized characteristics, such as race, attempts to address stigma aim to remove its effects on the patient rather than remove the characteristic itself. Thus, if it is assumed that people will continue to have diverse body sizes, solutions should be sought that will allow surgeons to safely operate on individuals of all sizes, including fat individuals.
We recognize that, in addition to explicit, intentional BMI thresholds, de facto BMI thresholds for surgery also exist,9 which include technical difficulties and equipment limitations. We hope these barriers to care can be resolved through innovation and investment in equipment, such as operating tables and longer tools suited for patients at high weights or with more tissue.57 Bariatric surgery specialists can model learning proper techniques and using equipment for safer operations.57 Examples from colorectal surgery include alternative incision sites and use of prophylactic mesh when there is more visceral fat.44 Preoperative radiographic imaging for flap surgeries, such as phalloplasty, can inform procedure decision making and planning.7,58,59 BMI can also be used to identify cases appropriate for less experienced surgeons.43
Access to GAS for fat trans people will not improve if BMI thresholds continue to bar patients from care without critical consideration of their use. BMI lacks the nuance to fully inform surgical candidacy. While still acknowledging the discriminatory origins of BMI, we believe its usefulness remains due to its ubiquity in the existing surgical outcomes literature. In a vacuum where no weight stigma exists, BMI is a helpful metric for data collection and procedural decision making, as well as for innovation of novel solutions in surgery for fat individuals. The problem is that BMI can enable and reinforce weight stigma, and that is what we must avoid. When assessing surgical candidacy, the risks associated with high BMI must be weighed against the benefits of GAS, which can be life-changing and sometimes even lifesaving.
References
-
Defining adult overweight & obesity. Centers for Disease Control and Prevention. Reviewed June 3, 2022. Accessed December 24, 2022. https://www.cdc.gov/obesity/basics/adult-defining.html
- Martinson TG, Ramachandran S, Lindner R, Reisman T, Safer JD. High body mass index is a significant barrier to gender-confirmation surgery for transgender and gender-nonbinary individuals. Endocr Pract. 2020;26(1):6-15.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014.
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015-3021.
- Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192(31):E875-E891.
- Winfield RD, Reese S, Bochicchio K, Mazuski JE, Bochicchio GV. Obesity and the risk for surgical site infection in abdominal surgery. Am Surg. 2016;82(4):331-336.
- Saiganesh H, Stein DE, Poggio JL. Body mass index predicts operative time in elective colorectal procedures. J Surg Res. 2015;197(1):45-49.
-
Castle E, Blasdel G, Shakir NA, Zhao LC, Bluebond-Langner R. Weight stigma mitigating approaches to gender-affirming genital surgery. Plast Aesthet Res. 2022;9(3):20.
- Hedenstierna G, Tokics L, Scaramuzzo G, Rothen HU, Edmark L, Öhrvik J. Oxygenation impairment during anesthesia: influence of age and body weight. Anesthesiology. 2019;131(1):46-57.
- Brownstone LM, DeRieux J, Kelly DA, Sumlin LJ, Gaudiani JL. Body mass index requirements for gender-affirming surgeries are not empirically based. Transgend Health. 2021;6(3):121-124.
- Phelan SM, Burgess DJ, Yeazel MW, Hellerstedt WL, Griffin JM, van Ryn M. Impact of weight bias and stigma on quality of care and outcomes for patients with obesity. Obes Rev. 2015;16(4):319-326.
-
Paine EA. “Fat broken arm syndrome”: negotiating risk, stigma, and weight bias in LGBTQ healthcare. Soc Sci Med. 2021;270:113609.
-
NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. National Heart, Lung, and Blood Institute; 1998. Report 98-4083. Assessed June 15, 2023. https://www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/2007154166-as-ob_gdlns.pdf
-
Tomiyama AJ. Weight stigma is stressful. A review of evidence for the cyclic obesity/weight-based stigma model. Appetite. 2014;82:8-15.
- Saguy AC, Ward A. Coming out as fat: rethinking stigma. Soc Psychol Q. 2011;74(1):53-75.
-
Strings S. Fearing the Black Body: The Racial Origins of Fat Phobia. New York University Press; 2019.
-
Strings S. Obese Black women as “social dead weight”: reinventing the “diseased Black woman.” Signs. 2015;41(1):107-130.
- Thorpe RJ Jr, Parker LJ, Cobb RJ, Dillard F, Bowie J. Association between discrimination and obesity in African-American men. Biodemography Soc Biol. 2017;63(3):253-261.
- Bidstrup H, Brennan L, Kaufmann L, de la Piedad Garcia X. Internalised weight stigma as a mediator of the relationship between experienced/perceived weight stigma and biopsychosocial outcomes: a systematic review. Int J Obes (Lond). 2022;46(1):1-9.
- Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156(7):611-618.
- Pittelkow EM, Duquette SP, Rhamani F, Rogers C, Gallagher S. Female-to-male gender-confirming drainless mastectomy may be safe in obese males. Aesthet Surg J. 2020;40(3):NP85-NP93.
-
Rothenberg KA, Gologorsky RC, Hojilla JC, et al. Gender-affirming mastectomy in transmasculine patients: does obesity increase complications or revisions? Ann Plast Surg. 2021;87(1):24-30.
-
Ives GC, Fein LA, Finch L, et al. Evaluation of BMI as a risk factor for complications following gender-affirming penile inversion vaginoplasty. Plast Reconstr Surg Glob Open. 2019;7(3):e2097.
- Berry MG, Curtis R, Davies D. Female-to-male transgender chest reconstruction: a large consecutive, single-surgeon experience. J Plast Reconstr Aesthet Surg. 2012;65(6):711-719.
- Frederick MJ, Berhanu AE, Bartlett R. Chest surgery in female to male transgender individuals. Ann Plast Surg. 2017;78(3):249-253.
- Donato DP, Walzer NK, Rivera A, Wright L, Agarwal CA. Female-to-male chest reconstruction: a review of technique and outcomes. Ann Plast Surg. 2017;79(3):259-263.
- McEvenue G, Xu FZ, Cai R, McLean H. Female-to-male gender affirming top surgery: a single surgeon’s 15-year retrospective review and treatment algorithm. Aesthet Surg J. 2017;38(1):49-57.
- Kääriäinen M, Salonen K, Helminen M, Karhunen-Enckell U. Chest-wall contouring surgery in female-to-male transgender patients: a one-center retrospective analysis of applied surgical techniques and results. Scand J Surg. 2017;106(1):74-79.
- van de Grift TC, Elfering L, Bouman MB, Buncamper ME, Mullender MG. Surgical indications and outcomes of mastectomy in transmen: a prospective study of technical and self-reported measures. Plast Reconstr Surg. 2017;140(3):415e-424e.
- Knox ADC, Ho AL, Leung L, et al. A review of 101 consecutive subcutaneous mastectomies and male chest contouring using the concentric circular and free nipple graft techniques in female-to-male transgender patients. Plast Reconstr Surg. 2017;139(6):1260e-1272e.
- Gallagher S, Rahmani F, Russell A, Duquette S. A drain-free technique for female-to-male gender affirmation chest surgery decreases morbidity: outcomes from 306 consecutive masculoplasties. Ann Plast Surg. 2019;83(1):15-21.
- Watanabe T, Sakurai T, Mukai Y, Kimata Y, Namba Y. Risk factors for postoperative hematoma after chest wall contouring for female-to-male transsexuals: a clinical study. Acta Med Okayama. 2019;73(5):441-447.
-
Stein MJ, Grigor E, Hardy J, Jarmuske M. Surgical and patient-reported outcomes following double incision and free nipple grafting for female to male gender affirmation: does obesity make a difference? J Plast Reconstr Aesthet Surg. 2021;74(8):1743-1751.
-
Naides AI, Schultz JJ, Shulzhenko NO, Keith JD. Chest masculinization technique and outcomes in 72 double-incision chest-contouring procedures with free nipple grafting. Plast Reconstr Surg Glob Open. 2021;9(3):e3459.
-
Rifkin WJ, Robinson IS, Kloer C, et al. Gender-affirming mastectomy: comparison of periareolar and double incision patterns. Plast Reconstr Surg Glob Open. 2022;10(5):e4356.
- Gaither TW, Awad MA, Osterberg EC, et al. Postoperative complications following primary penile inversion vaginoplasty among 330 male-to-female transgender patients. J Urol. 2018;199(3):760-765.
- Ascha M, Massie JP, Morrison SD, Crane CN, Chen ML. Outcomes of single stage phalloplasty by pedicled anterolateral thigh flap versus radial forearm free flap in gender confirming surgery. J Urol. 2018;199(1):206-214.
- Wirthmann AE, Majenka P, Kaufmann MC, et al. Phalloplasty in female-to-male transsexuals by Gottlieb and Levine’s free radial forearm flap technique-a long-term single-center experience over more than two decades. J Reconstr Microsurg. 2018;34(4):235-241.
- Watanabe T, Namba Y, Kimata Y. Flap selection algorithm based on the body mass index for phalloplasty in female-to-male transgender: techniques and outcomes. J Reconstr Microsurg Open. 2021;6(2):e57-e62.
- Spennato S, Ederer IA, Borisov K, et al. Radial forearm free flap phalloplasty in female-to-male transsexuals—a comparison between Gottlieb and Levine’s and Chang and Hwang’s technique. J Sex Med. 2022;19(4):661-668.
-
Bordas N, Stojanovic B, Bizic M, Szanto A, Djordjevic ML. Metoidioplasty: surgical options and outcomes in 813 cases. Front Endocrinol (Lausanne). 2021;12:760284.
-
Uchida T, Higure T, Kawakami M, et al. What factors affect the operative time of robot-assisted laparoscopic radical prostatectomy? Surg Endosc. 2021;35(8):4436-4443.
- Aquina CT, Rickles AS, Probst CP, et al. Visceral obesity, not elevated BMI, is strongly associated with incisional hernia after colorectal surgery. Dis Colon Rectum. 2015;58(2):220-227.
- van der Sluis WB, de Bruin RJM, Steensma TD, Bouman MB. Gender-affirmation surgery and bariatric surgery in transgender individuals in the Netherlands: considerations, surgical techniques and outcomes. Int J Transgend Health. 2022;23(3):355-361.
- Chang LD, Buncke G, Slezak S, Buncke HJ. Cigarette smoking, plastic surgery, and microsurgery. J Reconstr Microsurg. 1996;12(7):467-474.
- MacLean PS, Wing RR, Davidson T, et al. NIH working group report: innovative research to improve maintenance of weight loss. Obesity (Silver Spring). 2015;23(1):7-15.
-
Sequeira GM, Miller E, McCauley H, Eckstrand K, Rofey D. Impact of gender expression on disordered eating, body dissatisfaction and BMI in a cohort of transgender youth. J Adolesc Health. 2017;60(2)(suppl 1):S87.
- Linsenmeyer WR, Katz IM, Reed JL, Giedinghagen AM, Lewis CB, Garwood SK. Disordered eating, food insecurity, and weight status among transgender and gender nonbinary youth and young adults: a cross-sectional study using a nutrition screening protocol. LGBT Health. 2021;8(5):359-366.
-
Bacon L, Aphramor L. Weight science: evaluating the evidence for a paradigm shift. Nutr J. 2011;10:9.
- McPhail D, Orsini M. Fat acceptance as social justice. CMAJ. 2021;193(35):E1398-E1399.
- Zubarevich A, Szczechowicz M, Zhigalov K, et al. Surgical redo mitral valve replacement in high-risk patients: the real-world experience. J Card Surg. 2021;36(9):3195-3204.
- Moonesinghe SR, Mythen MG, Grocott MPW. High-risk surgery: epidemiology and outcomes. Anesth Analg. 2011;112(4):891-901.
-
Caballero B. Humans against obesity: who will win? Adv Nutr. 2019;10(suppl 1):S4-S9.
-
Talumaa B, Brown A, Batterham RL, Kalea AZ. Effective strategies in ending weight stigma in healthcare. Obes Rev. 2022;23(10):e13494.
-
Calogero RM, Tylka TL, Mensinger JL. Scientific weightism: a view of mainstream weight stigma research through a feminist lens. In: Roberts TA, Curtin N, Duncan LE, Cortina LM, eds. Feminist Perspectives on Building a Better Psychological Science of Gender. Springer International Publishing; 2016:9-28.
-
Recommendations for facilities performing bariatric surgery. American College of Surgeons. September 1, 2001. Accessed December 23, 2022. https://www.facs.org/about-acs/statements/recommendations-for-facilities-performing-bariatric-surgery/
- Sinove Y, Kyriopoulos E, Ceulemans P, Houtmeyers P, Hoebeke P, Monstrey S. Preoperative planning of a pedicled anterolateral thigh (ALT) flap for penile reconstruction with the multidetector CT scan. Handchir Mikrochir Plast Chir. 2013;45(4):217-222.
- Annen AW, Heston AL, Dugi DD III, et al. Masculinizing genital surgery: an imaging primer for the radiologist. AJR Am J Roentgenol. 2020;214(1):W27-W36.



