Abstract
Ethically evaluating prescription of weight loss pharmaceuticals for adolescents classified by body mass index (BMI) as obese requires reconsideration of how medicine’s overreliance on BMI as a diagnostic criterion supports a weight normative approach to health. This commentary on a case suggests that weight loss is not a safe, effective, or permanent method of health promotion. The unknown extent of pharmacotherapeutics’ risks to adolescents in addition to the controvertible benefits of weight loss ethically preclude their prescription, despite scientific consensus to fight obesity by prescribing weight reduction.
Case
M is a student at Sunnyvale High School. At 16 years old, they are currently enrolled in an intensive health behavior and lifestyle treatment (IHBLT) at the local county hospital. During the pandemic, their body mass index (BMI) increased from 28 to 30, making them a candidate for liraglutide, a glucagon-like peptide 1 (GLP-1) analogue approved by the US Food and Drug Administration (FDA) in 2020 as a weight loss medication in adolescents. As M’s primary care physician, Dr B recommends liraglutide as an additional means for preventing M’s becoming an obese adult with comorbidities.
Commentary
Responding to the title question requires not only evaluating the risks and benefits of pharmacotherapy (particularly in adolescents), but also closely examining weight loss as a health goal. Present clinical practice is “weight normative”1 in emphasizing weight and weight loss to define health and well-being. There is no more obvious manifestation of this practice than the continued use of BMI to define health status. BMI is based on the ratio of weight in kilograms to height in meters squared and is currently used as the identifying obesity indicator,2,3 although its value does not reflect significant considerations of the obesity disease state, including peripheral and visceral adiposity, body composition, and metabolic indices.2,4
Calling attention to how questionable BMI is as a litmus test for obesity are patients classified as overweight or obese (BMI ≥ 25) whose weight, when evaluated by physical and metabolic fitness, does not necessarily pose a risk to their health.5,6 Research on the “obesity paradox”4,7 and “metabolically healthy obesity”8,9,10 substantiates the existence of this incongruence between the expected and actual health or risk status associated with an obesity diagnosis. Additionally, the clinical distress of obesity can exist in bodies that do not match the expected phenotype of obesity (ie, fat), which are described in literature as thin-fat phenotype, normal weight obesity, metabolic obesity, and metabolically unhealthy non-obese.11
An obesity diagnosis that is defined by weight categorization (BMI ≥ 30) is problematic not only because the diagnostic accuracy of BMI is debatable, but also because it arguably leaves the impression that if too much weight is the problem, then less of it is the solution. Based on data collected from 2017 to 2020, the Centers for Disease Control and Prevention (CDC) determined the obesity prevalence to be 22.2% in adolescents aged 12 to 19.12,13 The increasing rate of adolescents classified as obese by BMI is often cited as a public crisis on the national and global level,14 with calls to address this crisis through interventions aimed at weight loss, including IHBLT programs, pharmacotherapeutics, and surgeries.15 Yet a 2015 Lancet publication found that no country has yet resolved its obesity epidemic despite these purported weight loss solutions.16
Updated Pediatric Guidelines
The 2023 publication of the American Academy of Pediatrics (AAP) Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents with Obesity17 refocused attention on pharmacotherapeutic inducement of weight loss in adolescents, with the recommendation being changed from watchful waiting to offering pharmacotherapeutics to those ages 12 years and older as an adjunct to behavioral and lifestyle obesity treatment.18
What should clinicians consider when deciding upon weight loss pharmacotherapy in obesity management? The US Food and Drug Administration (FDA) 2007 “Guidance for the Clinical Evaluation of Weight-Control Drugs” articulates these considerations:
Lifestyle modification, consisting of changes in patterns of dietary intake, exercise, and other behaviors, is considered the cornerstone of overweight and obesity management. Because all drug and biological therapies impose some risk for adverse events, the use of a weight management product should be contemplated only after a sufficient trial of lifestyle modification has failed and the risks of excess adiposity and the anticipated benefits of weight loss are expected to outweigh the known and unknown risks of treatment with a particular weight-management product.19
Crudely summarized, lifestyle modifications must fail before clinicians consider pharmacotherapies as an adjunct. Side effects of pharmacotherapies for weight loss must be less risky than untreated excess adiposity, understood to be a risk factor for or marker of weight-related disease states. BMI stratification is utilized as a proxy for identifying excess adiposity (ie, obesity). Therefore, failure of lifestyle modifications can be understood as lack of BMI shift or, less stringently, weight reduction.
In this case, despite participation in an IHBLT, M experienced an increase in BMI, which means the program failed to inhibit or reverse weight progression. Dr B might anticipate that weight loss via pharmaceutical intervention would reduce excess adiposity and therefore resolve M’s current obesity diagnosis while reducing the likelihood of adult obesity with its associated comorbidities—benefits that would outweigh the expected risks of liraglutide. Dr B’s introduction of pharmacotherapy would be in line with pediatric obesity treatment algorithms that recommend tiered comprehensive multidisciplinary interventions,20,21 including the AAP guidelines.17 These treatment approaches are rooted in the premise that, for reasons of current health and future risk, weight in excess of certain clinical parameters (ie, BMI ≥ 25) is bad and that weight loss is both achievable and good for health—so much so that it merits induction by biomedical means.
The remainder of this commentary will examine the feasibility as well as the benefits of weight loss cited to justify pharmaceutical interventions, weight loss pharmaceuticals for adolescents, and the implications of weight loss encouragement as a means of achieving health with the goal of promoting greater understanding of the dialogue surrounding adolescent obesity22 and weight loss pharmacotherapeutics. (In what follows, the phrase “classified as obese” will be used in lieu of “obese adolescents” to call attention to the role of BMI in weight-related disease diagnoses and not as an endorsement of person-first language23 in medicine’s discussion of obesity.)
Use of BMI in Pediatric Populations
Dual energy x-ray absorptiometry scans are relatively accurate measures of adiposity,17 but they are impractical on a large scale,24 prompting the use of BMI as a proxy for measuring adiposity in body composition. Studies have shown that BMI has high specificity but relatively low sensitivity for detecting excess adiposity.25,26 In pediatric obesity studies, BMI z-score (BMIz) is often used as a standardized measurement because BMIz tends to remain the same as a child gains weight while maturing into adulthood.27 However, in overweight and obese youth, BMIz is a poor predictor of relative body fat and therefore unlikely to be accurate if used to monitor adiposity changes resulting from weight management interventions.24,28,29
In pediatric populations, no risk-stratified BMI cutoffs exist akin to adult BMI classifications, which the World Health Organization and National Institutes of Health developed in 1995 and 1998, respectively, based on data relating BMI to mortality risk.19 For adolescents, overweight and obesity is often defined by the 85th and 95th percentile, respectively, of the BMI-for-age in the sex-specific reference population; race/ethnicity is not taken into consideration.30 These cutoff points in children, as well as the terminology of overweight and obese, lack “strong evidence for any precise” consensus,27 perhaps indicating that these are nosological entities31 borrowed from adult medicine for their familiarity rather than their accuracy. Even if BMI/z could be used to accurately assess and longitudinally monitor adiposity composition in pediatric populations, the inflection point between adiposity being a biological necessity and a threat to health is not clearly defined, particularly in pediatric populations during development.29
Realities of Weight Loss
Adolescents classified as obese generally remain so in adulthood, with a 2016 meta-analysis finding that “around 80% of obese adolescents will still be obese in adulthood.”32 The probability of attaining normal weight for people with an obesity classification is low, with one study of adults classified as overweight or obese reporting the annual probability over a maximum 9-year follow-up to be “1 in 210 for men and 1 in 124 for women [with simple obesity], increasing to 1 in 1290 for men and 1 in 677 for women with morbid obesity.”33 Cochrane systematic reviews evaluating diet, physical activity, and surgical and pharmaceutical interventions found low-quality evidence of their effectiveness for weight management in adolescent or childhood obesity, as well as a lack of safety data, particularly with regard to long-term effects.34,35,36,37,38
For pediatric populations, there is no general consensus on what constitutes clinically meaningful weight loss (usually estimated to be 5% to 10%39,40 of body weight in adults) or how long the weight loss should29 be sustained in order for an intervention to be considered successful (which is similarly undecided in adults39). Only a few studies have tracked long-term weight loss persistence,39 and even fewer have done so in pediatric populations.41,42 An oft-quoted 1959 study estimated that 95% of people who lose weight gain it back long term.43 More recent studies confirm weight regain as being par for the course,44 including a 2001 meta-analysis of 29 long-term studies, which found that, on average, more than 80% of lost weight was regained within 5 years.45 Weight loss, if any, tends to be insufficient to move patients into the non-obesity BMI range: IHBLTs reduce BMI an estimated 1% to 3% in children,17 bariatric surgery reduces BMI approximately 26% to 29% long-term46 (with a majority of adolescents having reduced bone mass and nutritional deficiencies),47 and anti-obesity drugs in adults taken for at least 12 months induce a 2.9% to 6.8% weight reduction from baseline.48 The Look AHEAD study found that, after 8 years of continuous intervention, only 50.3% and 35.7% of the participants in the intensive lifestyle intervention and diabetes support and education groups, respectively, lost at least 5% of their initial weight (the overall initial average BMI was 36).49
The putative benefits of weight loss are generally positive by clinical standards,40 but they tend to be either dependent on weight loss permanence (eg, cardiometabolic improvements50,51) or relatively independent of weight loss. Lifestyle interventions can be effective in “improving obesity-related comorbidities (eg, insulin resistance, hypertension, hyperlipidemia, fatty liver disease, and exercise capacity) even in the absence of sustained weight loss.”52 A 2022 cohort study concluded that only 15.6% to 46.8% of the association between weight loss strategies and type 2 diabetes risk could be attributed to weight changes.53 It could be concluded that perhaps it is the weight loss strategy itself, rather than the weight loss,54 that begets the desired health outcomes.
Despite the dubious feasibility of attaining and maintaining long-term clinically significant weight loss and the indication that weight loss may not be key to addressing health concerns linked to obesity, some studies persist in recommending weight loss, suggesting that even temporary weight loss is potentially valuable.55 However, repeated weight loss attempts56,57,58 with accompanying weight gain, otherwise known as weight cycling, lead to increased risk of disordered eating,59 higher mortality due to all causes and to cardiovascular disease (CVD),60 higher comorbidity of CVD and hypertension,60 worse cardiometabolic and lipid measures,61 and escalated weight regain.62,63
Our understanding of psychological outcomes in weight loss-oriented treatment is limited because existing studies rarely report mental health or well-being outcomes, and those that do show mixed results.64,65,66,67 Remarkably, merely perceiving failure in weight control (perhaps due to weight regain or not achieving expected weight loss in the first place) is associated with negative psychological outcomes.68,69 Weight treatments for adolescent are particularly ripe for concerns about disordered eating behaviors (DEBs) and eating disorders (EDs).70 The onset of EDs is usually during adolescence,70 with weight stigma and dieting being common precipitating factors.71,72 Studies have found that roughly 40% of overweight adolescent girls and 20% of overweight adolescent boys exhibit DEBs.72,73 Adolescents classified as obese tend to have low self-esteem, negative self-evaluation, and high body dissatisfaction,74 placing them at higher risk for restrictive eating escalating into a disorder.75 The AFINOS and AVENA studies found the odds of adolescents classified as overweight developing EDs to be 2.5 to 4.9 times higher, respectively, than their peers categorized as normal weight.76
Weight loss is not essential to improving comorbidities and tends to be minimal and impermanent, with repeated attempts being typical
Treatment for DEBs/EDs in adolescents classified as obese or overweight is regularly delayed by the pervasive perception of weight loss as invariably good rather than as a canary signaling clinical danger.77,78 Less than 6% of people with EDs are medically diagnosed as underweight,79,80 and the weight history of a significant portion of those presenting for ED treatment (37% to 41%) includes an overweight or obesity classification.73,81 As of 2022, screening tools for EDs in adolescents with obesity are still not validated,71,82,83 which undermines implementation of any recommendations (such as those in section IX.B.3. of the AAP guideline)17 for DEBs/EDs assessments in this targeted population prior to and during implementing weight management strategies like pharmacotherapy.
Pharmaceuticals have been described as the prescription for fat people of what is diagnosed as disordered in thin people84—that is, the acceptable biomedicalization of the pathological: skipping meals (anorectics), diet pills (pharmacotherapeutics themselves), laxatives (orlistat), and vomiting (a common glucagon-like peptide 1-related adverse effect).85,86 Considering the vulnerability to and higher prevalence of DEBs/EDs in adolescents classified as overweight or obese, the explicit valuing of weight loss as a success metric in pharmaceutical obesity management is worrisome in that it aligns with a weight normative approach to health,1 which has been shown to increase the risk for weight cycling and DEBs/EDs.87,88,89
In summary, weight loss is not essential to improving comorbidities and tends to be minimal and impermanent, with repeated attempts being typical. Particularly in adolescents, making weight loss the primary aim of health interventions (including pharmaceuticals) exacerbates the likelihood of destructive outcomes such as DEBs/EDs and weight cycling.
FDA and Weight Loss Pharmaceuticals
The FDA evaluates weight loss pharmacotherapies, or anti-obesity medications, by their mean and categorical efficacy, as defined in “Guidance for Industry: Developing Products for Weight Management” (originally published in 1996 as “Guidance for the Clinical Evaluation of Weight-Control Drugs”).19 After 1 year of treatment, the difference in the mean weight loss between the active-product and placebo groups must be statistically significant and at least 5% (ie, mean efficacy). Alternatively, after 1 year of treatment, at least 35% of participants in the active-product group should lose at least 5% of their initial weight, the proportion who lose at least 5% of their initial weight “is approximately double the proportion in the placebo-treated group, and the difference between groups is statistically significant” (ie, categorical efficacy). The 5% benchmark was selected because research before 1996 indicated that weight reductions of 5% to 10% improved metrics such as blood pressure, indexes of glycemia, and high-density lipoprotein cholesterol. 90,91
The FDA itself notes that “pediatric-specific adverse events are unlikely to be detected in development programs that are limited in size and duration” and that “long-term effects of drug treatment in children can include impacts on development, growth, and/or maturation of organ/system function.”92 Additionally, the FDA evaluation does not include a period of pharmaceutical cessation, hampering our understanding of weight loss permanence and regain associated with treatment timelines.
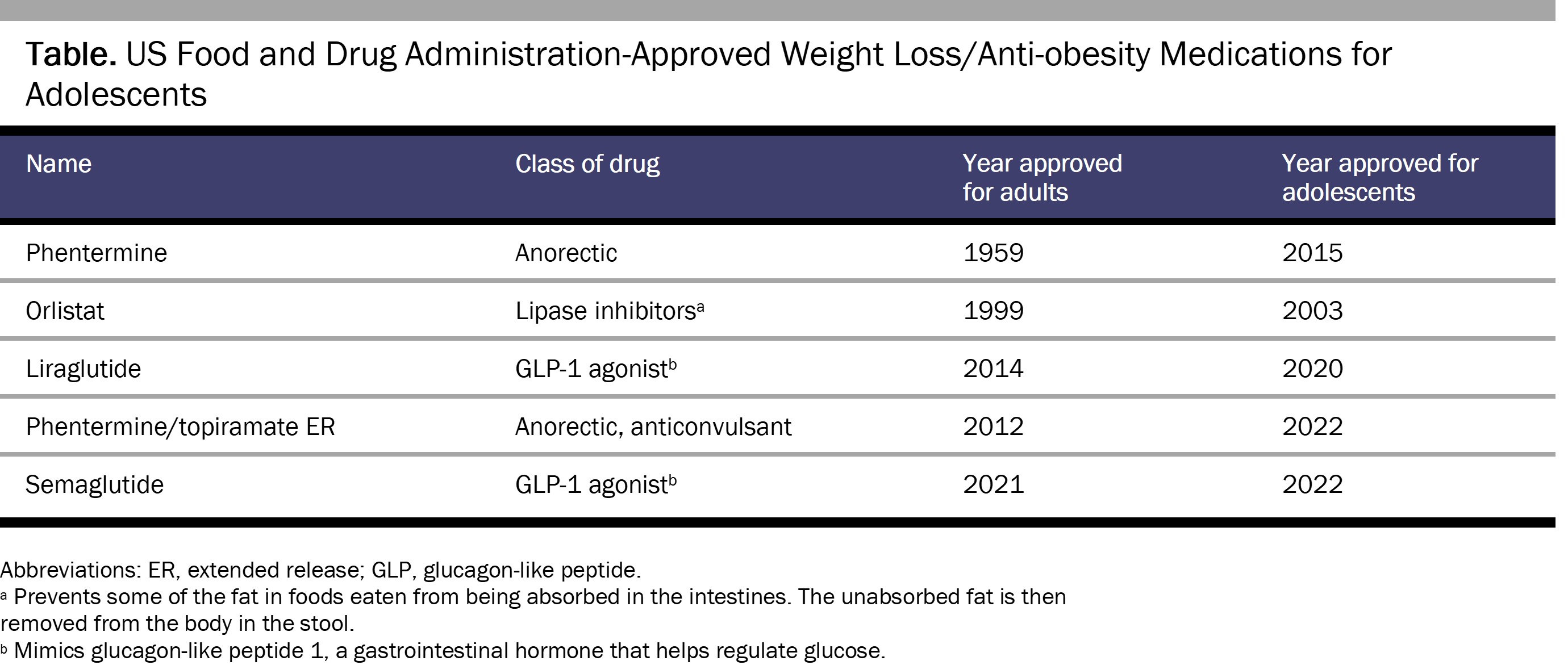
As of February 2023, there are 4 FDA-approved weight loss drugs for adolescents older than 12 years of age: orlistat,93 liraglutide,94 semaglutide,95 and phentermine/topiramate extended-release capsules.96 Additionally, phentermine is permitted for individuals older than 16 years of age for 12 weeks or less (see Table).97,98 In the near future, the FDA is likely to approve the diabetes drug tirzepatide for adolescent weight loss.99 Practitioners also use other medications off-label, including bupropion/naltrexone, topiramate, lisdexamfetamine, and (most commonly) metformin.100,101
The history of weight loss/anti-obesity medications is littered with recalls,102 including of fenfluramine, dexfenfluramine, sibutramine 103 and, most recently, lorcaserin,104 due to postmarket phase discovery of risks ranging from primary pulmonary hypertension91,105 to cardiac valvulopathy106 to cancer.104 The more recently approved medications, such as liraglutide and semaglutide, should arguably be safer given the availability of safety information on their active compounds, which have been used for years in other formulations.107 However, the application of weight loss/anti-obesity pharmacotherapies to adolescents is still relatively new and therefore the risk profile is relatively underdetermined.
Weight Loss Medications for Adolescents
Weight loss medications specifically approved for adolescents are relatively new, with off-label prescriptions being the norm.108 Currently, pharmaceuticals are intended as an adjunct rather than as monotherapy17 after lifestyle and behavioral medications, such as IHBLTs, fail to produce weight loss.19
IHBLTs are intended to serve as a first-line approach to reduce the frequency of pharmaceutical prescription, thereby avoiding unnecessary exposure to harm.109 However, numerous studies document that IHBLT and other similar interventions do not result in weight loss for the majority of adult49,110,111 and adolescent112,113 participants long-term, with the result that, for most, the lifestyle intervention will be deemed a failure, prompting clinicians to recommend pharmaceutical intervention.17,19 IHBLT programs vary in their characteristics114 while similarly suffering high patient attrition,112,113, 115 possibly because the intense time and resource investment required negatively impact participation.17 Without uniform quality standards for IHBLTs, it is difficult to determine whether weight loss failure is due to treatment resistance or nonadherence or to poor intervention quality. With IHBLTs tending toward failure and pharmaceuticals being relatively undemanding to implement, weight loss pharmacotherapeutics may rapidly become the dominant treatment modality for adolescent obesity.
In its 2023 practice guidelines, the AAP concedes that evidence on using pharmaceuticals to aid weight/BMI reduction is currently insufficient.17 There are a relatively small number of completed clinical trials, which tend to collect limited information116 and be inadequately powered due to small sample sizes.117 Available data indicate that average weight loss is typically minimal: 1.5% BMI reduction from baseline after 12 months’ treatment with orlistat,118 4.1% BMI reduction from baseline after 6 months’ treatment with phentermine,52 4.29% BMI reduction from baseline after treatment with liraglutide for 56 weeks,55 and 16.1% BMI reduction from baseline after treatment with semaglutide for 68 weeks.119 Common side effects (eg, nausea, vomiting, gastrointestinal distress)120 cause a noteworthy number of participant treatment discontinuations during clinical trials: 17.1% for orlistat vs 11.7% for the placebo group,121 13.8% for liraglutide vs 6.8% for the placebo group,55 and 14.8% for semaglutide vs 4.3% for the placebo group.119
The history of weight loss medications indicates that adverse drug reactions (including those resulting in a box warning or withdrawal) are not fully understood until the postmarket phase.122,123,124 Studies assessing FDA approval of new drugs125,126 find that approval is increasingly based on “fewer, smaller, or less rigorous pivotal trials,”127 thereby shifting the burden of evidence of adverse effects to the post-approval period.128 A study of all drugs approved by the FDA between 2001 and 2010 found that more than a third were affected by a postmarket safety event (withdrawals, boxed warnings, safety communications).124 With regard to weight loss drugs specifically, there is a dearth of long-term studies of the effects of weight loss pharmaceuticals in adolescents,129 and, as a result, our knowledge of their risks is lacking. Extrapolating potential side effects in adolescents from studies with adults130 is insufficient because, as the AAP notes, adolescents are undergoing growth and pubertal development, which can “alter the kinetics, end-organ responses, and toxicities” of the pharmaceutical in question.131 Health care practitioners will need to consider that early adoption of weight loss medications means that significant side effects—particularly long-term or developmental ones—will likely be identified in their patients during postmarket surveillance. This possibility is ethically troubling, given that many adolescents who will initially qualify for pharmaceutical intervention due to BMI belong to minoritized or under-resourced populations,17 raising concerns about the justness of these adolescents bearing the brunt of side effect discovery during the postmarket phase without more significant investment to discover these issues during the clinical trial phase.
Research on weight regain in adolescents after pharmaceutical discontinuation is scarce, but the emerging evidence is consistent with the pattern found in adults.51,55 Weight regain and loss of “attendant health benefits”17,51 after pharmaceutical cessation are mentioned as reasons to switch framing obesity from an acute121 to a “chronic relapsing progressive disease process”132 requiring continuous treatment. This push to extend treatment timelines indefinitely should spark concerns not only about our limited understanding of long-term side effects of weight loss medications in adolescents, but also about the potential impact of out-of-pocket cost on medication adherence.133 Medication adherence is low in adolescents to begin with, and even lower for those with long-term conditions.134,135 Many private insurers follow the lead of Medicare, which, outside of Advantage plans, does not cover anti-obesity medications, leaving patients to pay hundreds of dollars a month out of pocket or risk weight regain.136,137 Inconsistent use could result from these access challenges, inadvertently exposing adolescents to the dangers of weight cycling.
The nonprofit Obesity Action Coalition (OAC) is currently pushing for the passage of the Treat and Reduce Obesity Act of 2021, which would expand Medicare benefits for IHBLT-type programs and expand coverage for FDA-approved chronic weight management medications.138,139 Top corporate partners of the OAC are Novo Nordisk® (semaglutide) and Eli Lilly (tirzepatide),140 both of which stand to make a fortune with the prescription of weight loss and anti-obesity pharmaceuticals for obesity diagnosed by what could be considered an indiscriminate standard—BMI.
In summary, the threshold for initial prescription of weight loss medications is low, given how failure is defined for lifestyle modifications. Pharmaceutical interventions induce modest weight loss at best (frequently with side effects) that requires persistent usage to maintain. Long-term side effects of such interventions in adolescents—especially on development—have arguably not been sufficiently established for adequate risk assessment. What few studies there are examining pharmaceutical safety and efficacy in adolescents tend to be small and inadequately powered.
Conclusion
Should pharmaceuticals be used as a weight loss intervention for adolescents classified as obese? There is no disputing that pharmaceuticals are an essential part of clinical practice, but as a result of sparse investigation and overvaluing of weight loss, physicians might be inaccurately assessing the benefits as outweighing the risks in prescribing pharmaceuticals to induce weight loss. There is no general consensus for what constitutes a healthy BMI or clinically significant weight loss in adolescents. What weight loss that does occur is typically transient, not enough to shift BMI categorization, and not necessary to produce desired health outcomes. The pursuit of weight control,141 a tactic of weight-normative health promotion, is likely to result in—but is not limited to—weight dissatisfaction142,143,144 and stigma,145 DEBs/EDs, and weight cycling. All of these consequences are linked to worse health outcomes and further weight gain—the very opposite of the intended effect. The risks of continuous pharmaceutical treatment in adolescents in order to potentially stabilize weight loss are not yet known. The unknown extent of pharmacotherapeutics’ risks to adolescents for the controvertible benefits of weight loss ethically precludes their prescription, despite the scientific consensus to fight obesity by prescribing weight reduction.146
BMI and weight as defining clinical metrics distort our conception of what is required for health, justifying a dogged commitment to the erasure of fatness as health promotion rather than the interrogation of the biological, social, environmental, and economic factors impacting bodies.147 Pharmacological interventions might eventually become a key, safe, and effective component of the comprehensive care of patients navigating obesity. However, the justification of risks—particularly for adolescents—will depend on the congruence of the intended outcome with health reconceptualized as more than just anti-fatness. This reconceptualization will require scientific and ethical examination of the evidence, narratives,148 and assumptions influencing how medicine understands and deems desirable goals of health.149 Weight-neutral and weight-inclusive approaches1,87,88,150,151,152,153 provide insight into actualizing a clinical practice in which weight status—rather than being the definitive standard—is just one factor informing our understanding and pursuit of health.
References
-
Tylka TL, Annunziato RA, Burgard D, et al. The weight-inclusive versus weight normative approach to health: evaluating the evidence for prioritizing well-being over weight loss. J Obes. 2014;2014:983495.
-
De Lorenzo A, Gratteri S, Gualtieri P, Cammarano A, Bertucci P, Di Renzo L. Why primary obesity is a disease? J Transl Med. 2019;17(1):169.
-
Obesity. World Health Organization. Accessed September 6, 2022. https://www.who.int/health-topics/obesity
-
Donini LM, Pinto A, Giusti AM, Lenzi A, Poggiogalle E. Obesity or BMI paradox? Beneath the tip of the iceberg. Front Nutr. 2020;7:53.
-
Reynolds G. Why exercise is more important than weight loss for a longer life. New York Times. September 29, 2021. Accessed September 6, 2022. https://www.nytimes.com/2021/09/29/well/move/exercise-weight-loss-longer-life.html
- Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71-82.
- Flegal KM, Ioannidis JPA. The obesity paradox: a misleading term that should be abandoned. Obesity (Silver Spring). 2018;26(4):629-630.
-
Vukovic R, Dos Santos TJ, Ybarra M, Atar M. Children with metabolically healthy obesity: a review. Front Endocrinol (Lausanne). 2019;10:865.
- Videira-Silva A, Freira S, Fonseca H. Metabolically healthy overweight adolescents: definition and components. Ann Pediatr Endocrinol Metab. 2020;25(4):256-264.
- Tomiyama AJ, Hunger JM, Nguyen-Cuu J, Wells C. Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005-2012. Int J Obes (Lond). 2016;40(5):883-886.
-
Kapoor N. Thin fat obesity: the tropical phenotype of obesity. In: Feingold KR, Anawalt B, Blackman MR, et al, eds. Endotext. Updated March 14, 2021. Accessed March 3, 2023. http://www.ncbi.nlm.nih.gov/books/NBK568563/
-
Childhood obesity facts. Centers for Disease Control and Prevention. Reviewed May 17, 2022. Accessed September 6, 2022. https://www.cdc.gov/obesity/data/childhood.html
-
Clinical growth charts. Centers for Disease Control and Prevention. Reviewed June 16, 2017. Accessed September 6, 2022. https://www.cdc.gov/growthcharts/clinical_charts.htm
- Abarca-Gómez L, Abdeen ZA, Hamid ZA, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627-2642.
-
Nicolucci A, Maffeis C. The adolescent with obesity: what perspectives for treatment? Ital J Pediatr. 2022;48:9.
- Roberto CA, Swinburn B, Hawkes C, et al. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet. 2015;385(9985):2400-2409.
-
Hampl SE, Hassink SG, Skinner AC, et al. Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics. 2023;151(2):e2022060640.
-
American Academy of Pediatrics issues its first comprehensive guideline on evaluating, treating children and adolescents with obesity. News release. American Academy of Pediatrics; January 9, 2023. Accessed February 9, 2023. https://www.aap.org/en/news-room/news-releases/aap/2022/american-academy-of-pediatrics-issues-its-first-comprehensive-guideline-on-evaluating-treating-children-and-adolescents-with-obesity/
-
Center for Drug Evaluation and Research. Guidance for industry: developing products for weight management. US Food and Drug Administration; 2007. Accessed April 4, 2023. https://www.fda.gov/media/71252/download
- Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164-S192.
-
Cuda SE, Censani M. Pediatric obesity algorithm: a practical approach to obesity diagnosis and management. Front Pediatr. 2019;6:431.
- Patterson M, Johnston J. Theorizing the obesity epidemic: health crisis, moral panic and emerging hybrids. Soc Theory Health. 2012;10(3):265-291.
-
Meadows A, Daníelsdóttir S. What’s in a word? On weight stigma and terminology. Front Psychol. 2016;7:1527.
-
Vanderwall C, Randall Clark R, Eickhoff J, Carrel AL. BMI is a poor predictor of adiposity in young overweight and obese children. BMC Pediatr. 2017;17(1):135.
- Simmonds M, Burch J, Llewellyn A, et al. The use of measures of obesity in childhood for predicting obesity and the development of obesity-related diseases in adulthood: a systematic review and meta-analysis. Health Technol Assess. 2015;19(43):1-336.
- Javed A, Jumean M, Murad MH, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity in children and adolescents: a systematic review and meta-analysis. Pediatr Obes. 2015;10(3):234-244.
-
Flegal KM, Ogden CL. Childhood obesity: are we all speaking the same language? Adv Nutr. 2011;2(2):159S-166S.
-
Vanderwall C, Eickhoff J, Randall Clark R, Carrel AL. BMI Z-score in obese children is a poor predictor of adiposity changes over time. BMC Pediatr. 2018;18(1):187.
- Cardel MI, Atkinson MA, Taveras EM, Holm JC, Kelly AS. Obesity treatment among adolescents. JAMA Pediatr. 2020;174(6):609-617.
- Firman N, Boomla K, Hudda MT, Robson J, Whincup P, Dezateux C. Is child weight status correctly reported to parents? Cross-sectional analysis of National Child Measurement Programme data using ethnic-specific BMI adjustments. J Public Health (Oxf). 2020;42(4):e541-e550.
- Jutel A. The emergence of overweight as a disease entity: measuring up normality. Soc Sci Med. 2006;63(9):2268-2276.
- Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev. 2016;17(2):95-107.
- Fildes A, Charlton J, Rudisill C, Littlejohns P, Prevost AT, Gulliford MC. Probability of an obese person attaining normal body weight: cohort study using electronic health records. Am J Public Health. 2015;105(9):e54-e59.
- Gates A, Elliott SA, Shulhan-Kilroy J, Ball GDC, Hartling L. Effectiveness and safety of interventions to manage childhood overweight and obesity: an overview of Cochrane systematic reviews. Paediatr Child Health. 2020;26(5):310-316.
-
Ells LJ, Mead E, Atkinson G, et al. Surgery for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2015;(6):CD011740.
-
Axon E, Atkinson G, Richter B, et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2016;11(11): CD012436.
-
Al-Khudairy L, Loveman E, Colquitt JL, et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years. Cochrane Database Syst Rev. 2017;6(6):CD012691.
-
Brown T, Moore THM, Hooper L, et al. Do diet and physical activity strategies help prevent obesity in children (aged 0 to 18 years)? Cochrane Database Syst Rev. 2019;(7):CD001871.
-
Marchesini G, Montesi L, El Ghoch M, Brodosi L, Calugi S, Dalle Grave R. Long-term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab Syndr Obes. 2016;9:37-46.
-
Health benefits of weight loss. Obesity Evidence Hub. Updated April 16, 2020. Accessed March 1, 2023. https://www.obesityevidencehub.org.au/collections/impacts/health-benefits-of-weight-loss
-
Butryn ML, Wadden TA, Rukstalis MR, et al. Maintenance of weight loss in adolescents: current status and future directions. J Obes. 2010;2010:789280.
- Steinbeck KS, Lister NB, Gow ML, Baur LA. Treatment of adolescent obesity. Nat Rev Endocrinol. 2018;14(6):331-344.
- Stunkard A, McLaren-Hume M. The results of treatment for obesity: a review of the literature and report of a series. AMA Arch Intern Med. 1959;103(1):79-85.
- Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am. 2018;102(1):183-197.
- Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74(5):579-584.
- Ahn SM. Current issues in bariatric surgery for adolescents with severe obesity: durability, complications, and timing of intervention. J Obes Metab Syndr. 2020;29(1):4-11.
- Halloun R, Weiss R. Bariatric surgery in adolescents with obesity: long-term perspectives and potential alternatives. Horm Res Paediatr. 2022;95(2):193-203.
- Tak YJ, Lee SY. Anti-obesity drugs: long-term efficacy and safety: an updated review. World J Mens Health. 2021;39(2):208-221.
- Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: the Look AHEAD study. Obesity (Silver Spring). 2014;22(1):5-13.
- Vermeiren E, Bruyndonckx L, De Winter B, Verhulst S, Van Eyck A, Van Hoorenbeeck K. The effect of weight regain on cardiometabolic health in children with obesity: a systematic review of clinical studies. Nutr Metab Cardiovasc Dis. 2021;31(9):2575-2586.
- Wilding JPH, Batterham RL, Davies M, et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: the STEP 1 trial extension. Diabetes Obes Metab. 2022;24(8):1553-1564.
- Kühnen P, Biebermann H, Wiegand S. Pharmacotherapy in childhood obesity. Horm Res Paediatr. 2022;95(2):177-192.
-
Si K, Hu Y, Wang M, Apovian CM, Chavarro JE, Sun Q. Weight loss strategies, weight change, and type 2 diabetes in US health professionals: a cohort study. PLOS Med. 2022;19(9):e1004094.
-
Tomiyama AJ, Ahlstrom B, Mann T. Long-term effects of dieting: is weight loss related to health? Soc Pers Psychol Compass. 2013;7(12):861-877.
- Kelly AS, Auerbach P, Barrientos-Perez M, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. 2020;382(22):2117-2128.
- Santos I, Sniehotta FF, Marques MM, Carraça EV, Teixeira PJ. Prevalence of personal weight control attempts in adults: a systematic review and meta-analysis. Obes Rev. 2017;18(1):32-50.
-
Quinn DM, Puhl RM, Reinka MA. Trying again (and again): weight cycling and depressive symptoms in US adults. PLOS One. 2020;15(9):e0239004.
- Halali F, Lapveteläinen A, Aittola K, et al. Associations between weight loss history and factors related to type 2 diabetes risk in the Stop Diabetes study. Int J Obes (Lond). 2022;46(5):935-942.
- Rhee EJ. Weight cycling and its cardiometabolic impact. J Obes Metab Syndr. 2017;26(4):237-242.
-
Zou H, Yin P, Liu L, et al. Body-weight fluctuation was associated with increased risk for cardiovascular disease, all-cause and cardiovascular mortality: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2019;10:728.
- Kakinami L, Knäuper B, Brunet J. Weight cycling is associated with adverse cardiometabolic markers in a cross-sectional representative US sample. J Epidemiol Community Health. 2020;74(8):662-667.
- Saarni SE, Rissanen A, Sarna S, Koskenvuo M, Kaprio J. Weight cycling of athletes and subsequent weight gain in middle age. Int J Obes (Lond). 2006;30(11):1639-1644.
- Korkeila M, Rissanen A, Kaprio J, Sørensen TIA, Koskenvuo M. Weight-loss attempts and risk of major weight gain: a prospective study in Finnish adults. Am J Clin Nutr. 1999;70(6):965-975.
- Lowry KW, Sallinen BJ, Janicke DM. The effects of weight management programs on self-esteem in pediatric overweight populations. J Pediatr Psychol. 2007;32(10):1179-1195.
-
Jackson SE, Steptoe A, Beeken RJ, Kivimaki M, Wardle J. Psychological changes following weight loss in overweight and obese adults: a prospective cohort study. PLoS One. 2014;9(8):e104552.
-
Hoare E, Fuller-Tyszkiewicz M, Skouteris H, Millar L, Nichols M, Allender S. Systematic review of mental health and well-being outcomes following community-based obesity prevention interventions among adolescents. BMJ Open. 2015;5(1):e006586.
- Järvholm K, Bruze G, Peltonen M, et al. 5-year mental health and eating pattern outcomes following bariatric surgery in adolescents: a prospective cohort study. Lancet Child Adolesc Health. 2020;4(3):210-219.
- Bombak AE, Monaghan LF. Obesity, bodily change and health identities: a qualitative study of Canadian women. Sociol Health Illn. 2017;39(6):923-940.
-
Ju YJ, Han KT, Lee TH, Kim W, Park JH, Park EC. Association between weight control failure and suicidal ideation in overweight and obese adults: a cross-sectional study. BMC Public Health. 2016;16(1):259.
-
Golden NH, Schneider M, Wood C; Committee on Nutrition; Committee on Adolescence; Section on Obesity. Preventing obesity and eating disorders in adolescents. Pediatrics. 2016;138(3):e20161649.
- Chen C, Gonzales L. Understanding weight stigma in eating disorder treatment: development and initial validation of a treatment-based stigma scale. J Health Psychol. 2022;27(13):3028-3045.
- Neumark-Sztainer DR, Wall MM, Haines JI, Story MT, Sherwood NE, van den Berg PA. Shared risk and protective factors for overweight and disordered eating in adolescents. Am J Prev Med. 2007;33(5):359-369.
- Sim LA, Lebow J, Billings M. Eating disorders in adolescents with a history of obesity. Pediatrics. 2013;132(4):e1026-e1030.
-
Stabouli S, Erdine S, Suurorg L, Jankauskienė A, Lurbe E. Obesity and eating disorders in children and adolescents: the bidirectional link. Nutrients. 2021;13(12):4321.
- Haynos AF, Watts AW, Loth KA, Pearson CM, Neumark-Stzainer D. Factors predicting an escalation of restrictive eating during adolescence. J Adolesc Health. 2016;59(4):391-396.
-
Veses AM, Martínez-Gómez D, Gómez-Martínez S, et al; AVENA/AFINOS Study Groups. Physical fitness, overweight and the risk of eating disorders in adolescents. The AVENA and AFINOS studies. Pediatr Obes. 2014;9(1):1-9.
- Harrop EN, Mensinger JL, Moore M, Lindhorst T. Restrictive eating disorders in higher weight persons: a systematic review of atypical anorexia nervosa prevalence and consecutive admission literature. Int J Eat Disord. 2021;54(8):1328-1357.
-
Peebles R, Hardy KK, Wilson JL, Lock JD. Are diagnostic criteria for eating disorders markers of medical severity? Pediatrics. 2010;125(5):e1193-e1201.
- Flament MF, Henderson K, Buchholz A, et al. Weight status and DSM-5 diagnoses of eating disorders in adolescents from the community. J Am Acad Child Adolesc Psychiatry. 2015;54(5):403-411.e2.
- Duncan AE, Ziobrowski HN, Nicol G. The prevalence of past 12-month and lifetime DSM-IV eating disorders by BMI category in US men and women. Eur Eat Disord Rev. 2017;25(3):165-171.
- Sim LA, Lebow J, Billings M. Eating disorders in adolescents with a history of obesity. Pediatrics. 2013;132(4):e1026-e1030.
-
Jebeile H, Lister NB, Baur LA, Garnett SP, Paxton SJ. Eating disorder risk in adolescents with obesity. Obes Rev. 2021;22(5):e13173.
- House ET, Lister NB, Seidler AL, et al. Identifying eating disorders in adolescents and adults with overweight or obesity: a systematic review of screening questionnaires. Int J Eat Disord. 2022;55(9):1171-1193.
-
Metz LD. Eating disorders and malnutrition across the weight spectrum. Mosaic Comprehensive Care. February 26, 2020. Accessed May 22, 2023. https://mosaiccarenc.com/uncategorized/eating-disorders-malnutrition-across-weight-spectrum/
- Müller TD, Blüher M, Tschöp MH, DiMarchi RD. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov. 2022;21(3):201-223.
-
Gorgojo-Martínez JJ, Mezquita-Raya P, Carretero-Gómez J, et al. Clinical recommendations to manage gastrointestinal adverse events in patients treated with GLP-1 receptor agonists: a multidisciplinary expert consensus. J Clin Med. 2023;12(1):145.
-
Bacon L, Aphramor L. Weight science: evaluating the evidence for a paradigm shift. Nutr J. 2011;10:9.
- Hunger JM, Smith JP, Tomiyama AJ. An evidence-based rationale for adopting weight-inclusive health policy. Soc Issues Policy Rev. 2020;14(1):73-107.
- Doan N, Romano I, Butler A, Laxer RE, Patte KA, Leatherdale ST. Original quantitative research—weight control intentions and mental health among Canadian adolescents: a gender-based analysis of students in the COMPASS study. Health Promot Chronic Dis Prev Can. 2021;41(4):119-130.
- Colman E. Food and Drug Administration’s obesity drug guidance document: a short history. Circulation. 2012;125(17):2156-2164.
- Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann Intern Med. 2005;143(5):380-385.
-
Center for Biologics Evaluation and Research. E11(R1) addendum: clinical investigation of medicinal products in the pediatric population: guidance for industry. US Food and Drug Administration; 2017. Accessed March 3, 2023. https://www.fda.gov/media/101398/download
-
O’Connor A, Grady D. FDA moves to let drug treat obese teenagers. New York Times. December 16, 2003. Accessed April 3, 2023. https://www.nytimes.com/2003/12/16/us/fda-moves-to-let-drug-treat-obese-teenagers.html
-
FDA approves weight management drug for patients aged 12 and older. US Food and Drug Administration. December 4, 2020. Accessed April 3, 2023. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-weight-management-drug-patients-aged-12-and-older
-
FDA approves once weekly Wegovy® injection for the treatment of obesity in teens aged 12 years and older. News release. Novo Nordisk®; December 23, 2022. Accessed April 3, 2023. https://www.novonordisk-us.com/media/news-archive/news-details.html?id=151389
-
FDA approves treatment for chronic weight management in pediatric patients aged 12 years and older. US Food and Drug Administration. June 27, 2022. Accessed April 3, 2023. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-chronic-weight-management-pediatric-patients-aged-12-years-and-older#:~:text=The%20U.S.%20Food%20and%20Drug,or%20greater%20when%20standardized%20for
- Ryder JR, Kaizer A, Rudser KD, Gross A, Kelly AS, Fox CK. Effect of phentermine on weight reduction in a pediatric weight management clinic. Int J Obes (Lond). 2017;41(1):90-93.
- Lewis KH, Fischer H, Ard J, et al. Safety and effectiveness of longer-term phentermine use: clinical outcomes from an electronic health record cohort. Obesity (Silver Spring). 2019;27(4):591-602.
-
Sharma A. Novo targets teens with Wegovy as it heads off Lilly Mounjaro threat. Scrip. November 7, 2022. Accessed January 20, 2023. https://scrip.pharmaintelligence.informa.com/SC147324/Novo-Targets-Teens-With-Wegovy-As-It-Heads-Off-Lilly-Mounjaro-Threat
- Srivastava G, Fox CK, Kelly AS, et al. Clinical considerations regarding the use of obesity pharmacotherapy in adolescents with obesity. Obesity (Silver Spring). 2019;27(2):190-204.
- Singhal V, Sella AC, Malhotra S. Pharmacotherapy in pediatric obesity: current evidence and landscape. Curr Opin Endocrinol Diabetes Obes. 2021;28(1):55-63.
-
Onakpoya IJ, Heneghan CJ, Aronson JK. Post-marketing withdrawal of anti-obesity medicinal products because of adverse drug reactions: a systematic review. BMC Med. 2016;14(1):191.
-
Ault A. Anti-obesity drugs recalled from global market. Lancet. 1997;350(9081):867.
-
FDA requests the withdrawal of the weight-loss drug Belviq, Belviq XR (lorcaserin) from the market. US Food and Drug Administration. February 13, 2020. Accessed April 3, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-withdrawal-weight-loss-drug-belviq-belviq-xr-lorcaserin-market
-
Abenhaim L, Moride Y, Brenot F, et al; International Primary Pulmonary Hypertension Study Group. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. N Engl J Med. 1996;335(9):609-616.
- Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337(9):581-588.
-
Cummins E. The future of weight loss looks a lot like its past. Wired. February 15, 2023. Accessed February 20, 2023. https://www.wired.com/story/fda-weight-loss-drugs/
- Czepiel KS, Perez NP, Campoverde Reyes KJ, Sabharwal S, Stanford FC. Pharmacotherapy for weight loss in children, adolescents, and young adults: an evaluation of current on- and off- label usage. Pediatrics. 2021;147(3):180-181.
- LeBlanc ES, Patnode CD, Webber EM, Redmond N, Rushkin M, O’Connor EA. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults. JAMA. 2018;320(11):1172-1191.
-
Singh N, Stewart RA, Benatar JR. Intensity and duration of lifestyle interventions for long-term weight loss and association with mortality: a meta-analysis of randomised trials. BMJ Open. 2019;9(8):e029966.
-
Unick JL, Beavers D, Bond DS, et al; Look AHEAD Research Group. The long-term effectiveness of a lifestyle intervention in severely obese individuals. Am J Med. 2013;126(3):236-242.e2.
-
van de Pas KG, Lubrecht JW, Hesselink ML, Winkens B, van Dielen FM, Vreugdenhil AC. The effect of a multidisciplinary lifestyle intervention on health parameters in children versus adolescents with severe obesity. Nutrients. 2022;14(9):1795.
- Reinehr T, Widhalm K, l’Allemand D, Wiegand S, Wabitsch M, Holl RW. Two-year follow-up in 21,784 overweight children and adolescents with lifestyle intervention. Obesity (Silver Spring). 2009;17(6):1196-1199.
-
Bondyra-Wiśniewska B, Myszkowska-Ryciak J, Harton A. Impact of lifestyle intervention programs for children and adolescents with overweight or obesity on body weight and selected cardiometabolic factors—a systematic review. Int J Environ Res Public Health. 2021;18(4):2061.
- Danieles PK, Ybarra M, Van Hulst A, et al. Determinants of attrition in a pediatric healthy lifestyle intervention: the circuit program experience. Obes Res Clin Pract. 2021;15(2):157-162.
- González Bagnes MF, González C, Hirschler V, Di Girolamo G. Pharmacotherapeutic options in pediatric obesity: an urgent call for further research. Expert Opin Pharmacother. 2022;23(8):869-872.
-
Woodard K, Louque L, Hsia DS. Medications for the treatment of obesity in adolescents. Ther Adv Endocrinol Metab. 2020;11:2042018820918789.
- Chanoine J, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293(23):2873-2883.
- Weghuber D, Barrett T, Barrientos-Pérez M, et al. Once-weekly semaglutide in adolescents with obesity. N Engl J Med. 2022;387(24):2245-2257.
- Raman V, Gupta A, Ashraf AP, et al. Pharmacologic weight management in the era of adolescent obesity. J Clin Endocrinol Metab. 2022;107(10):2716-2728.
- Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches. Circulation. 2013;128(15):1689-1712.
-
Alomar M, Tawfiq AM, Hassan N, Palaian S. Post marketing surveillance of suspected adverse drug reactions through spontaneous reporting: current status, challenges and the future. Ther Adv Drug Saf. 2020;11:204209862093859.
-
Zhang X, Zhang Y, Ye X, Guo X, Zhang T, He J. Overview of phase IV clinical trials for postmarket drug safety surveillance: a status report from the Clinicaltrials.gov registry. BMJ Open. 2016;6(11):e010643.
- Downing NS, Shah ND, Aminawung JA, et al. Postmarket safety events among novel therapeutics approved by the US Food and Drug Administration between 2001 and 2010. JAMA. 2017;317(18):1854-1863.
- Downing NS, Aminawung JA, Shah ND, Krumholz HM, Ross JS. Clinical trial evidence supporting FDA approval of novel therapeutic agents, 2005-2012. JAMA. 2014;311(4):368-377.
-
Zhang AD, Puthumana J, Downing NS, Shah ND, Krumholz HM, Ross JS. Assessment of clinical trials supporting US Food and Drug Administration approval of novel therapeutic agents, 1995-2017. JAMA Netw Open. 2020;3(4):e203284.
-
Mitra-Majumdar M, Gunter SJ, Kesselheim AS, et al. Analysis of supportive evidence for US Food and Drug Administration approvals of novel drugs in 2020. JAMA Netw Open. 2022;5(5):e2212454.
- Darrow JJ, Avorn J, Kesselheim AS. FDA approval and regulation of pharmaceuticals, 1983-2018. JAMA. 2020;323(2):164-176.
-
Butryn ML, Wadden TA, Rukstalis MR, et al. Maintenance of weight loss in adolescents: current status and future directions. J Obes. 2010;2010:789280.
-
Center for Devices and Radiological Health. Ethical considerations for clinical investigations of medical products involving children: guidance for industry, sponsors, and IRBs. US Food and Drug Administration; 2022. Accessed April 4, 2023. https://www.fda.gov/media/161740/download
- Shaddy RE, Denne SC. Guidelines for the ethical conduct of studies to evaluate drugs in pediatric populations. Pediatrics. 2010;125(4):850-860.
-
Bray GA, Kim KK, Wilding JPH; World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715-723.
- Kaul S, Avila JC, Mehta HB, Rodriguez AM, Kuo YF, Kirchhoff AC. Cost-related medication nonadherence among adolescent and young adult cancer survivors. Cancer. 2017;123(14):2726-2734.
- Taddeo D, Egedy M, Frappier JY. Adherence to treatment in adolescents. Paediatr Child Health. 2008;13(1):19-24.
-
Badawy SM, Thompson AA, Kuhns LM. Medication adherence and technology-based interventions for adolescents with chronic health conditions: a few key considerations. JMIR Mhealth Uhealth. 2017;5(12):e202.
-
Noguchi Y. Obesity drugs’ promise now hinges on insurance coverage. NPR. July 6, 2021. Accessed February 3, 2023. https://www.npr.org/sections/health-shots/2021/07/06/1007772101/obesity-drugs-promise-now-hinges-on-insurance-coverage
-
Rabin RC. The doctor prescribed an obesity drug. Her insurance called it “vanity.” New York Times. May 31, 2022. Accessed February 3, 2023. https://www.nytimes.com/2022/05/31/health/obesity-drugs-insurance.html
-
Treat and Reduce Obesity Act of 2021, S 596, 117th Cong, 1st sess (2021-2022). Accessed April 3, 2023. https://www.congress.gov/bill/117th-congress/senate-bill/596/text
-
Treat and Reduce Obesity Act of 2021, HR 1577, 117th Cong, 1st sess (2021-2022). Accessed April 3, 2023. https://www.congress.gov/bill/117th-congress/house-bill/1577/text
-
Corporate partners. Obesity Action Coalition. Accessed April 3, 2023. https://www.obesityaction.org/corporate-partners/
- Doan N, Romano I, Butler A, Laxer RE, Patte KA, Leatherdale ST. Weight control intentions and mental health among Canadian adolescents: a gender-based analysis of students in the COMPASS study. Health Promot Chronic Dis Prev Can. 2021;41(4):119-130.
- Wirth MD, Blake CE, Hébert JR, Sui X, Blair SN. Chronic weight dissatisfaction predicts type 2 diabetes risk: Aerobic Center Longitudinal Study. Health Psychol. 2014;33(8):912-919.
-
Blake CE, Hébert JR, Duck-Chul L, et al. Adults with greater weight satisfaction report more positive health behaviors and have better health status regardless of BMI. J Obes. 2013;2013:291371.
- Bearman SK, Presnell K, Martinez E, Stice E. The skinny on body dissatisfaction: a longitudinal study of adolescent girls and boys. J Youth Adolesc. 2006;35(2):217-229.
- Puhl R, Suh Y. Health consequences of weight stigma: implications for obesity prevention and treatment. Curr Obes Rep. 2015;4(2):182-190.
- Bosomworth NJ. Relationship between weight loss and body image in obese individuals seeking weight loss treatment. Can Fam Physician. 2012;58(5):517-523.
-
Nobles J, Summerbell C, Brown T, Jago R, Moore T. A secondary analysis of the childhood obesity prevention Cochrane review through a wider determinants of health lens: implications for research funders, researchers, policymakers and practitioners. Int J Behav Nutr Phys Act. 2021;18(1):22.
-
Flegal KM. The obesity wars and the education of a researcher: a personal account. Prog Cardiovasc Dis. 2021;67:75-79.
-
Gard M, Wright J. Science and fatness. In: The Obesity Epidemic: Science, Morality and Ideology. Routledge; 2005:1-15.
-
Khasteganan N, Lycett D, Furze G, Turner AP. Health, not weight loss, focused programmes versus conventional weight loss programmes for cardiovascular risk factors: a systematic review and meta-analysis. Syst Rev. 2019;8(1):200.
- Miller GD, Nicklas BJ, Davis C, et al. Obesity treatment: weight loss versus increasing fitness and physical activity for reducing health risks. Clin Sports Med. 1999;18(2):485-503.
- Matheson EM, King DE, Everett CJ. Healthy lifestyle habits and mortality in overweight and obese individuals. J Am Board Fam Med. 2012;25(1):9-15.
- Barry VW, Baruth M, Beets MW, Durstine JL, Liu J, Blair SN. Fitness vs. fatness on all-cause mortality: a meta-analysis. Prog Cardiovasc Dis. 2014;56(4):382-390.


